|
| | Osimertinib mesylate Basic information |
| Product Name: | Osimertinib mesylate | | Synonyms: | AZD-9291 (Mesylate);N-[2-[[2-(Dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1H-indol-3-yl)-2-pyrimidinyl]amino]phenyl]-2-propenamide methanesulfonate (1:1);AZD-9291 mesylate N-[2-[[2-(Dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1H-indol-3-yl)-2-pyrimidinyl]amino]phenyl]-2-propenamide methanesulfonate (1:1);Osimertinib;N-[2-(2-dimethylaminoethylmethylamino)-4-methoxy-5-[[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino]phenyl]prop-2-enamide mesylate salt;Osimertinib Mesylate(AZD9291);Osimertinib mesylate;Mereletinib mesylate | | CAS: | 1421373-66-1 | | MF: | C29H37N7O5S | | MW: | 595.72 | | EINECS: | 200-064-1 | | Product Categories: | AZD09;API;1421373-66-1 | | Mol File: | 1421373-66-1.mol |  |
| | Osimertinib mesylate Chemical Properties |
| Melting point | >232°C (dec.) | | storage temp. | -20°C Freezer, Under inert atmosphere | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | color | Off-White to Yellow | | InChI | InChI=1S/C28H33N7O2.CH4O3S/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24;1-5(2,3)4/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32);1H3,(H,2,3,4) | | InChIKey | FUKSNUHSJBTCFJ-UHFFFAOYSA-N | | SMILES | S(O)(=O)(=O)C.CN1C2=CC=CC=C2C(C2C=CN=C(NC3C=C(NC(=O)C=C)C(N(C)CCN(C)C)=CC=3OC)N=2)=C1 |
| | Osimertinib mesylate Usage And Synthesis |
| Description | Osimertinib is active against exon 19 deletions, exon 21 mutations, and also the
exon 20 T790M mutations. It is preferentially selective for mutated EGFR, and
therefore toxicity at therapeutic doses is lower than for first- and second-generation
agents. Notably, osimertinib is able to cross the blood-brain barrier, making it active
against disease in the CNS. | | Uses | AZD-9291 Mesylate is a potent and selective epidermal growth factor receptor (EGFR) inhibitor. | | Definition | ChEBI: A methanesulfonate (mesylate) salt prepared from equimolar amounts of osimertinib and methanesulfonic acid. Used for treatment of EGFR T790M mutation positive non-small cell lung cancer. | | Indications | The collection of ibrutinib (Imbruvica(R), Pharmacyclics Inc.), afatinib, and osimertinib represents the small, yet expanding, group of covalent SMKIs. Ibrutinib is a non-receptor Bruton’s tyrosine kinase inhibitor approved for the treatment of relapsed chronic lymphocytic leukemia. Afatinib, approved for NSCLC in 2013 and squamous NSCLC in 2016, is a second-generation irreversible EGFR inhibitor that targets wild-type EGFR, the mutant T790M EGFR, and HER2. Osimertinib (AZD9291), which was approved by FDA in November 2015, is a third-generation irreversible EGFR inhibitor that selectively targets the mutant T790M EGFR. Rociletinib, which shares a high degree of structural similarity with that of osimertinib, is a promising covalent EGFR inhibitor developed by Clovis Oncology aimed for the treatment of patients with EGFR T790M-mutated NSCLC, until the company terminated its development in May 2016 following a negative vote fromthe FDA’sOncologic Drugs Advisory Committee. | | Side effects | Osimertinib toxicity is dose-dependent and is associated with fewer gastrointestinal
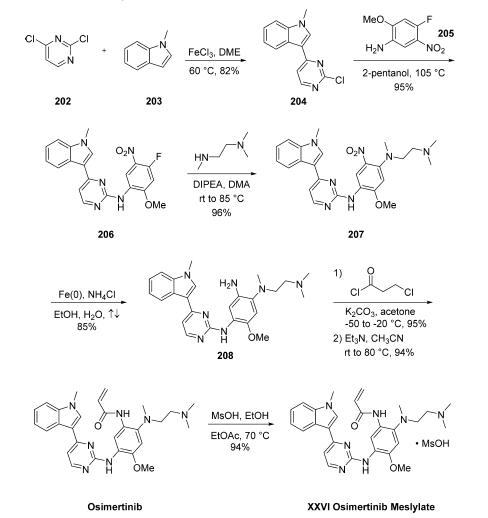
and dermatologic adverse events than with other approved EGFR TKIs. | | Synthesis | Friedel-Crafts arylation of commercial N-methylindole
(203) with commercial dichloropyrimidine 202 gave the 3-
pyrazinyl indole 204 in good yield. Subsequent SNAr with
nitroaniline 205 (available from a one-step nitration from the
commercially available des-nitroaniline) provided aminopyrazine
206. Next, SN
Ar reaction of 206 with N,N,N??-
trimethylated ethylenediamine delivered 207 in near quantitative
yield, and this was followed by nitro reduction with iron
under acidic conditions to give rise to the triaminated arene
208 in 85% yield. Because acrylates are notoriously difficult to
install directly due to their highly reactive nature and
propensity to polymerize, a clever two-step acylation/
elimination sequence was employed using 3-chloropropanoyl
chloride, and this was immediately followed by mesylate salt
formation, which furnished the osimertinib mesylate (XXVI) in
excellent yield. This seven-step process which derives from
readily available feedstock delivered the final product in nearly
57% overall yield from starting materials 202 and 203.
 |
| | Osimertinib mesylate Preparation Products And Raw materials |
|



