|
| | N,N-Dimethylbenzylamine Basic information |
| | N,N-Dimethylbenzylamine Chemical Properties |
| Melting point | -75 °C | | Boiling point | 183-184 °C765 mm Hg(lit.) | | density | 0.9 g/mL at 25 °C(lit.) | | vapor pressure | 2.4 hPa (20 °C) | | refractive index | n20/D 1.501(lit.) | | Fp | 130 °F | | storage temp. | Store below +30°C. | | solubility | water: soluble | | pka | pK1:9.02(+1) (25°C) | | form | Liquid | | color | Clear colorless to light yellow | | PH | 10 (10g/l, H2O, 20℃)(saturated solution) | | Odor | strong fish odor | | explosive limit | 0.9-6.3%(V) | | Water Solubility | 8 g/L (20 ºC) | | Sensitive | Air Sensitive | | BRN | 1099620 | | Stability: | Stable. Incompatible with strong acids, strong oxidizing agents. | | LogP | 1.98 at 25℃ | | CAS DataBase Reference | 103-83-3(CAS DataBase Reference) | | NIST Chemistry Reference | Benzenemethanamine, N,N-dimethyl-(103-83-3) | | EPA Substance Registry System | N,N-Dimethylbenzylamine (103-83-3) |
| Hazard Codes | C | | Risk Statements | 10-20/21/22-34-52/53 | | Safety Statements | 26-36-45-61 | | RIDADR | UN 2619 8/PG 2 | | WGK Germany | 2 | | RTECS | DP4500000 | | F | 10-13-23 | | Autoignition Temperature | 410 °C | | TSCA | Yes | | HazardClass | 8 | | PackingGroup | II | | HS Code | 29214980 | | Toxicity | LD50 orally in Rabbit: 290 mg/kg LD50 dermal Rat 695 mg/kg |
| Provider | Language |
|
BDMA
| English |
| | N,N-Dimethylbenzylamine Usage And Synthesis |
| Chemical Properties | Colorless to light yellow flammable liquid with ammonia odor. Soluble in ethanol, ether, insoluble in water. | | Uses | N,N-Dimethylbenzylamine was used in the synthesis of bis[(N,N-dimethylamino)benzyl] selenide. It has been used as catalyst during curing reaction of formulations of diglycidyl ether of bisphenol A and tetrahydrophthalic anhydride. It undergoes directed ortho metalation with butyl lithium. It reacts with methyl iodide to get ammonium salt, which is used as phase transfer catalysts. Further, it is used as a catalyst for the formation of polyurethane foams and epoxy resins. | | Preparation | 25% Aqueous Dimethylamine, 1088 grams
Benzyl Chloride, 126.6 grams
In the apparatus of Example 1, the benzyl chloride was added dropwise over a two-hour period to the amine (molar ratio 1 to 6) at a rate sufficient to maintain the temperature below 40°C. Stirring was continued at room temperature for an additional hour to insure completion of the reaction denoted by the equation below.
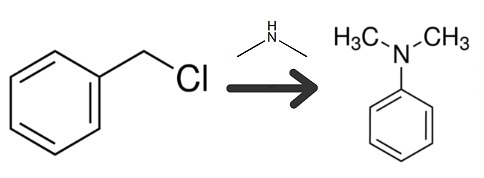
Thereafter the reaction mixture was cooled in a separatory funnel while standing in a refrigerator maintained at 5° C. and separated into two layers. The upper oily layer, weighing 111.5g, was removed and steam distilled until no further oleaginous component was observed in the distillate as it came over. The crude distillate was found to contain 103.5g of N,N-dimethylbenzylamine (76.1% of theory), 3.3g of dimethylamine and no quaternary salts. The dimethylamine was distilled off below 29°C under atmospheric pressure from the N,N-dimethylbenzylamine (bp 82°C/18mmHg).
| | Synthesis Reference(s) | The Journal of Organic Chemistry, 40, p. 531, 1975 DOI: 10.1021/jo00892a043
Synthetic Communications, 6, p. 539, 1976 DOI: 10.1080/00397917608063545 | | General Description | Benzyldimethylamine appears as a colorless to light yellow liquid with an aromatic odor. Slightly less dense than water and slightly soluble in water. Corrosive to skin, eyes and mucous membranes. Slightly toxic by ingestion, skin absorption and inhalation. Used in the manufacture of adhesives; dehydrohalogenating catalyst; corrosion inhibitor; acid neutralizer; potting compounds; cellulose modifier and quaternary ammonium compounds. | | Air & Water Reactions | Flammable. Slightly soluble in water. | | Reactivity Profile | N,N-Dimethylbenzylamine neutralizes acids on exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. May attack some plastics [USCG, 1999]. | | Health Hazard | Inhalation may be fatal as a result of spasm, inflammation and edema of the larynx and bronchi, chemical pneumonitis, and pulmonary edema. Symptoms of exposure may include burning sensation, coughing, wheezing, laryngitis, shortness of breath, headache, nausea, and vomitting. | | Fire Hazard | Special Hazards of Combustion Products: Toxic vapors are generated when heated. | | Safety Profile | Poison by ingestion.
Moderately toxic by inhalation and skin
contact. Corrosive. A severe eye and skin
irritant. Flammable when exposed to heat or
flame. When heated to decomposition it
emits toxic fumes of NOx | | Purification Methods | Dry the amine over KOH pellets and fractionate it over Zn dust in a CO2—free atmosphere. It has a pKa2 5 of 8.25 in 45% aqueous EtOH. Store it under N2 or in a vacuum. The picrate has m 94-95o, and the picrolonate has m 151o (from EtOH). [Skita & Keil Chem Ber 63 34 1930, Coleman J Am Chem Soc 55 3001 1933, Devereux et al. J Chem Soc 2845 1957.] The tetraphenyl borate salt has m 182-185o. [Crane Anal Chem 28 1794 1956, Beilstein 12 IV 2161.] |
| | N,N-Dimethylbenzylamine Preparation Products And Raw materials |
|



