|
| | macitentan Basic information |
| Product Name: | macitentan | | Synonyms: | N-[5-(4-Bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)-oxy]ethoxy]-4-pyrimidinyl]-N'-propylsulfami;macitentan;N-[5-(4-Bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N'-propylsulfamide;ACT 064992;ACT-064992;ACT-064992 / ACT064992;Macitentan, >=99%;5-(4-BROMOPHENYL)-6-[2-(5-BROMOPYRIMIDIN-2-YL)OXYETHOXY]-N-(PROPYLSULFAMOYL)PYRIMIDIN-4-AMINE | | CAS: | 441798-33-0 | | MF: | C19H20Br2N6O4S | | MW: | 588.27 | | EINECS: | 1308068-626-2 | | Product Categories: | Inhibitors;API;441798-33-0 | | Mol File: | 441798-33-0.mol |  |
| | macitentan Chemical Properties |
| Boiling point | 692.4±65.0 °C(Predicted) | | density | 1.675 | | storage temp. | Store at -20°C | | solubility | ≥24.4 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH | | form | solid | | pka | 4.99±0.50(Predicted) | | InChI | InChI=1S/C19H20Br2N6O4S/c1-2-7-26-32(28,29)27-17-16(13-3-5-14(20)6-4-13)18(25-12-24-17)30-8-9-31-19-22-10-15(21)11-23-19/h3-6,10-12,26H,2,7-9H2,1H3,(H,24,25,27) | | InChIKey | JGCMEBMXRHSZKX-UHFFFAOYSA-N | | SMILES | S(NC1C(C2=CC=C(Br)C=C2)=C(OCCOC2=NC=C(Br)C=N2)N=CN=1)(NCCC)(=O)=O |
| | macitentan Usage And Synthesis |
| Description | Macitentan (also known as ACT-064992) received US FDA approval in October 2013 for the treatment of pulmonary arterial hypertension (PAH) (WHO group I) to delay disease progression. Treatment options include phosphodiesterase type 5 inhibitors, prostacyclins, and the endothelin receptor antagonists bosentan and ambrisentan. Macitentan was discovered through SAR studies starting with the bosentan structure with three main goals: (1) to increase potency for both endothelin receptor A and B (ETA and ETB) subtypes; (2) to improve tissue distribution to reach the target receptors; and (3) to avoid bile salt transport inhibition. Starting with the bosentan sulfonamido-pyrimidinyl central core, potency was increased 10-fold via incorporation of a bromopyrimidinyl ethylene glycol ether, as found in the clinical endothelin antagonist, T-0201. An aryl ether in bosentan was replaced with the bromophenyl group in macitentan, and a substituent on the 2-position of the central pyrimidine was replaced with hydrogen. Several sulfonamides and alkyl sulfamates were explored, with the propylsulfamate providing the best combination of in vitro potency, especially for ETB antagonism, and in vivo efficacy. | | Originator | Actelion
Pharmaceuticals Ltd. (Switzerland) | | Uses | Macitentan is an endothelin receptor antagonist that is used in the therapy of pulmonary arterial hypertension (PAH). It also reduced hospitalization for PAH. Macitentan was approved for PAH by the United States Food and Drug Administration (FDA) in 2013. Macitentan has been associated with a low rate of serum enzyme elevations during therapy, but has yet to be implicated in cases of clinically apparent acute liver injury. | | Definition | ChEBI: Macitentan is a member of the class of sulfamides in which the two amino groups of sulfonamide are substituted by 5-(4-bromophenyl)-6-{2-[(5-bromopyrimidin-2-yl)oxy]ethoxy}pyrimidin-4-yl and propyl groups. An orphan drug used for the treatment of pulmonary arterial hypertension. It has a role as an endothelin receptor antagonist, an antihypertensive agent and an orphan drug. It is an organobromine compound, a member of pyrimidines, an aromatic ether, a ring assembly and a member of sulfamides. It is functionally related to an ethylene glycol and an ACT-132577. | | Brand name | Opsumit | | Clinical Use | Endothelin receptor antagonist:
Treatment of pulmonary arterial hypertension | | Synthesis | The preparation of the drug began with reaction of commercial
4-bromophenylacetate (112) with dimethylcarbonate (113) under
basic conditions to yield the malonate ester 114. Treatment
of this diester with sodium methoxide and formamidine
hydrochloride 115 provided the desired intermediate 4,6-
dihydroxypyrimidine as a tautomeric mixture; from this system,
dichloride 116 was generated in 60¨C80% yield upon treatment with
warm phosphorus oxychloride in N,N-dimethylaniline. Reaction of
116 with excess sulfonyl urea potassium salt 117 provided
chloropyrimidine 118 in high yield (83¨C93%). This was reacted
with bromochloropyrimidine 119 in an SNAr reaction to provide
macitentan (XV) in 88% yield.
Synthesis of sulfamide potassium salt 117 was accomplished
via sequential reaction of chlorosulfonyl isocyanate (120) with t-
BuOH and propylamine/Et3N to provide ester sulfamide 121,followed by Boc removal and treatment with potassium t-butoxide
to yield 117. This material could be isolated by trituration with
diethyl ether.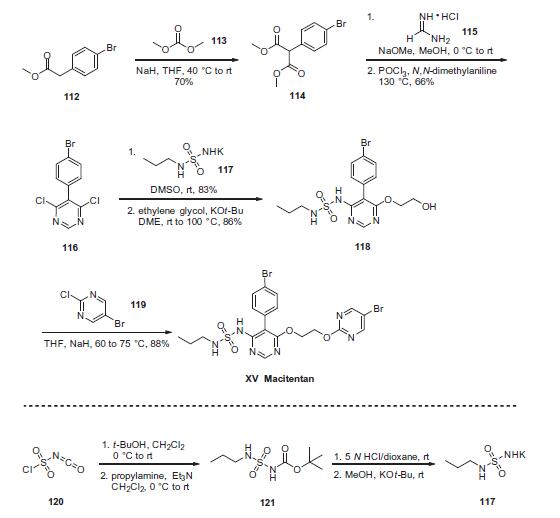
| | Mode of action | Macitentan is an orally available dual endothelin receptor (ETR) antagonist with potential antihypertensive and antineoplastic activity. Upon administration, macitentan and its metabolites block the binding of endothelin isoform 1 (ET-1) to type-A and type-B ETR on both the tumor cells and the endothelial cells in the tumor vasculature. This prevents ET-1 mediated signaling transduction which may decrease tumor cell proliferation, progression, and angiogenesis in tumor tissue. ET-1, a potent vasoconstrictor that plays an important role in inflammation and tissue repair, is, together with its receptors, overexpressed varyingly in many tumor cell types. | | references | [1]. marc iglarz, christoph binkert, keith morrison, et al. pharmacology of macitentan, an orally active tissue-targeting dual endothelin receptor antagonist. journal of pharmacology and experimental therapeutics, 2008, 327:736-745. |
| | macitentan Preparation Products And Raw materials |
|