|
| | Aclidinium bromide Basic information |
| Product Name: | Aclidinium bromide | | Synonyms: | (3R)-(2-Hydroxy-2,2-dithien-2-ylacetoxy)-1-(3-phenoxypropyl)-1-azoniabicyclo[2.2.2]octane bromide;Adiguanium bromide;1-Azoniabicyclo(2.2.2)octane, 3-((hydroxydi-2-thienylacetyl)oxy)-1-(3-phenoxypropyl)-, bromide, (3R)-;Unii-uqw7uf9N91;ACLIDINIUM BROMIDE INTERMEDIATES;ACLIDINIUM BROMIDE;LAS 34273;LAS-W 330 | | CAS: | 320345-99-1 | | MF: | C13H10BrN | | MW: | 564.56 | | EINECS: | 825-171-6 | | Product Categories: | Inhibitors | | Mol File: | 320345-99-1.mol |  |
| | Aclidinium bromide Chemical Properties |
| Melting point | 230 °C(Solv: acetonitrile (75-05-8)) | | storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C | | solubility | DMSO (Slightly, Heated), Methanol (Slightly) | | form | Solid | | color | White to Pale Orange |
| | Aclidinium bromide Usage And Synthesis |
| Description | In July 2012, aclidinium bromide was approved in the US and the EU for
long-term maintenance treatment of bronchospasm associated with chronic
obstructive pulmonary disorder (COPD).
Aclidinium bromide is in the latter category, acting as a selective
antagonist for the muscarinic M3 receptor. M3 receptors are localized
in airway smooth muscle, and are the primary subtype responsible for
bronchial and tracheal smooth muscle contraction. Muscarinic antagonists
are well-established bronchodilators that are effective for treating COPD,
but these agents have unwanted side effects if systemically absorbed. Systemic exposure can be limited by inhaled administration, which is the route
of delivery for aclidinium bromide. In addition, aclidinium bromide was
designed to undergo rapid hydrolysis in human plasma, providing inactive
acid and alcohol products, and reducing the potential for systemic side
effects. Aclidinium bromide was identified amongst a series of quaternary
ammonium (3R)-quinuclidinol esters as having the best combination of
high potency (M3 Ki=0.14 nM), long duration of action (29 h for 50%
reduction of therapeutic effect in a guinea pig bronchoconstriction model),
low oral absorption, and rapid plasma degradation. The synthesis of
aclidinium bromide was achieved by reaction of dimethyl oxalate with
2-thienylmagnesium bromide followed by treatment of the resulting methyl
ester with (3R)-quinuclidinol in the presence of sodium hydride. Quaternization of the amine was achieved by treatment with 3-phenoxypropyl
bromide to give aclidinium bromide. | | Originator | Almirall (Spain) | | Uses | Aclidinium Bromide inhibits human muscarinic AChR M1, M2, M3, M4 and M5 with Ki of 0.1 nM, 0.14 nM, 0.14 nM, 0.21 nM and 0.16 nM, respectively | | Uses | Aclidinium Bromide is a novel long-acting antimuscarinic bronchodilator in phase II clinical trials for the treatment of chronic obstructive pulmonary disease. | | Definition | ChEBI: A quaternary ammonium salt that is the bromide salt of aclidinium. A muscarinic acetylcholine M3 receptor antagonist, for the long-term maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease (COPD). | | Brand name | Tudorza Pressair? (US)
Eklira?/Bretaris? Genuair? (EU) | | Clinical Use | Aclidinium bromide was approved by the U. S. Food and Drug Administration (FDA) in July 2012
for the treatment of chronic obstructive pulmonary disease (COPD). Marketed by Forest
Pharmaceuticals, aclidinium bromide selectively binds to five human muscarinic receptors (M1-M5), and
posesses a subnanomolar binding affinity for these particular targets. Administered by inhalation, this
medicine has demonstrated favorable onset and duration of action, and its safety profile is an
improvement over competitor therapies. | | Synthesis | No manufacturing route has been disclosed to date, the most scalable published synthesis is described in the scheme. Dimethyl oxalate (1) was initially
treated with two equivalents of Grignard 2 to give bis-thiophenoate 3 in 36% yield. Subsequent
transesterification with (R)-quinuclidinol (4) gave rise to the quinuclidine-containing ester 5 in 50%
yield. Aclidinium bromide (I) could be accessed by two different methods involving bromoalkyl phenyl
ether 6?aan excess of bromide in the presence of an acetonitrile/chloroform mixture gave the drug in 89% isolated yield, or with fewer equivalents of electrophile (1.25 eq) during exposure to refluxing
acetophenone has reportedly delivered (I) quantitatively on multi-gram scale.17 From commercial 2, the multi-gram synthesis of Aclidinium bromide (I) was completed in 17.8% over three steps. 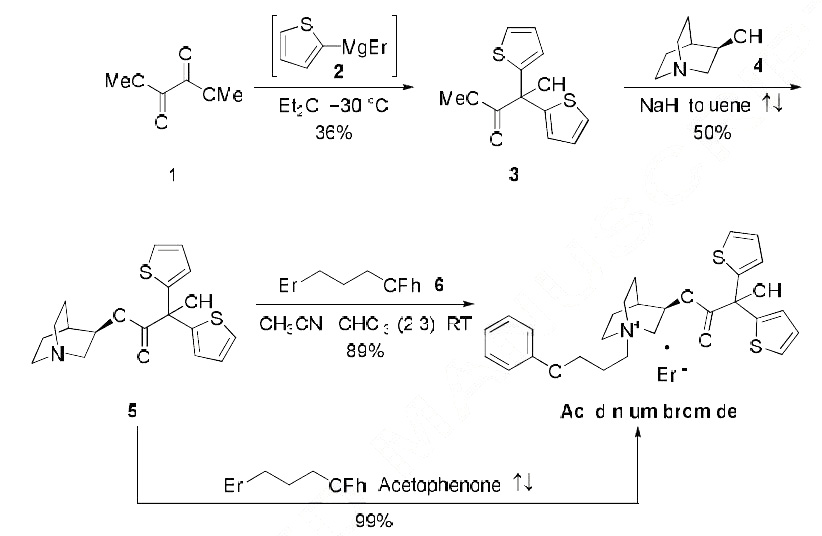
| | references | [1] gavaldà a1, ramos i2, carcasona c3, calama e4, otal r5, montero jl6, sentellas s7, aparici m8, vilella d9, alberti j10, beleta j11, miralpeix m12. the in vitro and in vivo profile of aclidinium bromide in comparison with glycopyrronium bromide. pulm pharmacol ther. 2014 aug;28(2):114-21. |
| | Aclidinium bromide Preparation Products And Raw materials |
|