|
| | N-(tert-Butyldimethylsilyl)-N-methyl-trifluoroacetamide Basic information |
| | N-(tert-Butyldimethylsilyl)-N-methyl-trifluoroacetamide Chemical Properties |
| Boiling point | 172-175 °C(lit.) | | density | 1.036 g/mL at 25 °C(lit.) | | refractive index | n20/D 1.402(lit.) | | Fp | 127 °F | | storage temp. | 2-8°C | | solubility | sol most aprotic organic solvents. | | form | Liquid | | pka | -1.50±0.70(Predicted) | | Specific Gravity | 1.023 | | color | Clear colorless to slightly yellow | | Water Solubility | decomposes | | Sensitive | Moisture Sensitive | | Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents | | BRN | 3606546 | | CAS DataBase Reference | 77377-52-7(CAS DataBase Reference) |
| | N-(tert-Butyldimethylsilyl)-N-methyl-trifluoroacetamide Usage And Synthesis |
| Chemical Properties | clear colorless to slightly yellow liquid | | Physical properties | bp 168–170°C; d 1.12 g cm?3. | | Uses | N-tert-Butyldimethylsilyl-N-methyltrifluoroacetamide with 1% tert-butyldimethylchlorosilane is commonly used for the preparation of N-tert-butyldimethylsilyl ethanolamines resulting from the hydrolysis of nitrogen mustards. It is also used for selective O-silylation of N,O-diacylhydroxyl amines. | | Uses | N-(tert-Butyldimethylsilyl)-N-methyl-trifluoroacetamide is a silylating agent used in the derivatization of various organic compounds (such as amino acids) for gas chromatogography-mass spectrometry (GC-MS) analysis.
| | Uses | In the
presence of 1% of t-butyldimethylchlorosilane as the catalyst,
N-(t-butyldimethylsilyl)-N-methyltrifluoroacetamide (BSMTFA)
functions as an extremely reactive t-butyldimethylsilylating
reagent for alcohols, amines, carboxylic acids, and thiols. Silylation
is generally completed within 5 min at 25°C in acetonitrile.
This amide is more reactive than N-(t-butyldimethylsilyl)-N-methylacetamide.
In the protection of a hydroxy group, the resultant tbutyldimethylsilyl
(TBDMS) ethers are stable under the conditions
for acetate saponification and hydrogenation.These silyl
ethers also remain intact towards the Jones reagent and Wittig
reagents. The TBDMS ethers are approx. 10 times more stable
against hydrolysis than the corresponding trimethylsilyl (TMS)
ethers.Selective removal of the TBDMS group can be accomplished
by use of dilute acetic acid or tetra-n-butylammonium
fluoride in THF at 25°C.
Silylation of ketones by use ofBSMTFAoccurs in triethylamine
and DMF at 40–60°C to give the corresponding silyl enol ethers
in good to excellent yields (eq 1).In addition, silyl ether formation
takes place in N-hydroxysuccinimide (88% yield) and Nhydroxypyrrole
(99% yield) by use of BSMTFA in THF.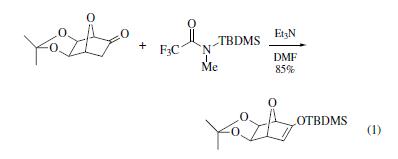 | | Preparation | obtained in 91% yield by reaction of
N-methyl-2,2,2-trifluoroacetamide (1.0 equiv) in benzene and
acetonitrile (1:1 v/v) with NaH (1.0 equiv) and then with
t-BuMe2SiCl (1.2 equiv) at 4°C. | | Definition | ChEBI: An N-silyl compound that is N-methyltrifluoroacetamide in which the amide nitrogen is replaced by a tert-butyldimethylsilyl group. | | General Description | N-tert-Butyldimethylsilyl-N-methyltrifluoroacetamide is a silylating reagent which replaces the active hydrogen with tert-Butyldimethylsilyl group. The tert-Butyldimethylsilyl derivatives are found to be more resistant to hydrolysis and stable compared to trimethylsilyl (TMS) derivatives. This reagent is suitable for GC-MS analysis since it produces mass spectra, which can be easily interpreted. It is widely used for the silylation of different alcohols, thiols, phenols, carboxylic acids, amines, and amides. |
| | N-(tert-Butyldimethylsilyl)-N-methyl-trifluoroacetamide Preparation Products And Raw materials |
|