|
| | DIMETHYLKETENE METHYL TRIMETHYLSILYL ACETAL Basic information |
| | DIMETHYLKETENE METHYL TRIMETHYLSILYL ACETAL Chemical Properties |
| Boiling point | 35 °C15 mm Hg(lit.) | | density | 0.858 g/mL at 25 °C(lit.) | | refractive index | n20/D 1.415(lit.) | | Fp | 58 °F | | storage temp. | Inert atmosphere,Room Temperature | | solubility | freely sol organic solvents. | | form | clear liquid | | color | Colorless to Almost colorless | | Specific Gravity | 0.858 | | Water Solubility | Hydrolyzes in water. | | Sensitive | Moisture Sensitive | | Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents | | BRN | 1362893 | | Stability: | Moisture and Acid Sensitive | | CAS DataBase Reference | 31469-15-5(CAS DataBase Reference) | | EPA Substance Registry System | Silane, [(1-methoxy-2-methyl-1-propenyl)oxy]trimethyl- (31469-15-5) |
| Hazard Codes | Xi | | Risk Statements | 10-36/37/38 | | Safety Statements | 16-26-36 | | RIDADR | UN 3271 3/PG 3 | | WGK Germany | 3 | | F | 10-21 | | TSCA | Yes | | HazardClass | 3 | | PackingGroup | III | | HS Code | 29319090 |
| | DIMETHYLKETENE METHYL TRIMETHYLSILYL ACETAL Usage And Synthesis |
| Chemical Properties | Clear colorless liquid | | Physical properties | bp 35 °C/15 mmHg; d 0.858 g cm?3. | | Uses | 1-Methoxy-2-methyl-1-(trimethylsilyloxy)
propene is widely used as functional equivalent of enolate of methyl isobutyrate; ester enolate
surrogate in electrophilic reactions including alkylation, aldol
reaction, Michael reaction, initiator for group transfer
polymerization of acrylates, nitroarylation, oxidation,
dimerization, and cycloadditions. | | Uses | 1-Methoxy-2-methyl-1-(trimethylsiloxy)propene is used in the synthesis of chiral β-lactams by reacting with (S)-alkylidene(1-arylethyl)amines in the presence of titanium tetrachloride. It acts as a catalyst or initiator in the group-transfer polymerization. It is also used as a versatile reagent in conjugate addition4 and aldol reactions. | | Preparation | The title compound is a prototypical ketene silyl acetal
(KSA) that can been prepared by either of the two most commonly
employed methods: (a) deprotonation of the |á-hydrogen of
an ester followed by silylation (1),16 and (b) metal-catalyzed
hydrosilylation of |á,|?-unsaturated esters(2). 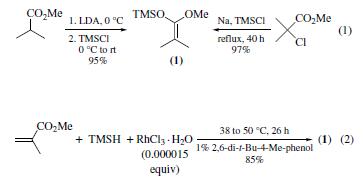
| | Purification Methods | Add Et2O, wash with cold H2O, dry (Na2SO4), filter, evaporate Et2O, and distil the oily residue in a vacuum. [Ainsworth et al. J Organometal Chem 46 59 1972.] |
| | DIMETHYLKETENE METHYL TRIMETHYLSILYL ACETAL Preparation Products And Raw materials |
|