|
| | Indacaterol Maleate Basic information |
| Product Name: | Indacaterol Maleate | | Synonyms: | 5-[(1R)-2-[(5,6-Diethyl-2,3-dihydro-1H-inden-2-yl)aMino]-1-hydroxyethyl]-8-hydroxy-2(1H)-quinolinone Maleic Acid;Indacaterol Maleic Acid Salt;Onbrez;QAB 149 Maleic Acid;5-[(1R)-2-[(5,6-Diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl]-8-hydroxy-2(1H)-quinolinone (2Z)-2-butenedioate;2(1H)-Quinolinone, 5-[(1R)-2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)aMino]-1-hydroxyethyl] -8-hydroxy-, (2Z)-2-butenedioate (1:1) (salt);2(1H)-Quinolinone, 5-[(1R)-2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl]-8-hydroxy-, (2Z)-2-butenedioate (1:1);ndacaterol Maleate | | CAS: | 753498-25-8 | | MF: | C28H32N2O7 | | MW: | 508.57 | | EINECS: | 691-329-4 | | Product Categories: | Agonist;Amines;Aromatics;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;API | | Mol File: | 753498-25-8.mol |  |
| | Indacaterol Maleate Chemical Properties |
| Melting point | 195-197°C (dec.) | | storage temp. | Refrigerator | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | color | White to Pale Beige |
| | Indacaterol Maleate Usage And Synthesis |
| Chemical Properties | White Solid | | Uses | A long-acting β2 adrenoreceptor agonist and bronchodilator, for the treatment of asthma and chronic obstructive pulmonary disease. | | Definition | ChEBI: A maleate salt obtained by reaction of indacaterol with one equivalent of maleic acid. Used for treatment of chronic obstructive pulmonary disease. | | Clinical Use | Indacaterol is a b-adrenoceptor agonist currently approved in
Europe as Onbrez, and is marketed by Novartis. It needs to be
taken only once a day, unlike competitors formoterol and salmeterol.
These drugs are used in the treatment of chronic obstructive
pulmonary disease (COPD) and asthma. Onbrez is administered via
an aerosol formulation through a dry powder inhaler. A Phase III
trial published in July 2010 suggested that indacaterol is significantly
more effective than twice-daily formoterol in improving
FEV1 and reduces the need for rescue medication. | | Synthesis | The synthesis
of indacaterol relies on the union of the dihydroindeneamine
region and the quinolinol region of the molecule. Preparation of
the dihydroindene unit of indacaterol was reported by researchers
at Novartis in 2006 and is summarized in the scheme.
2,3-Dihydro-1H-inden-2-amine (59) was protected as its trifluoroacetamide
61 and was followed by Friedel¨CCrafts alkylation with
acetylchloride to give 62. Hydrogenative carbonyl reduction of this
unsymmetrical dihydroindene provided amide 63. An iterative Friedel¨CCrafts acylation/hydrogenation sequence was used to install
the second ethyl group, giving rise to trifluoroacetamide 64.
Basic hydrolysis to remove the trifluoroacetamide functionality,
followed by salt formation by means of gaseous HCl furnished
the dihydroindene amine 65. The synthesis of the remaining
portion of the molecule starts from 8-hydroxyquinoline (66).
Friedel¨CCrafts alkylation with acetylchloride and trichloroaluminum
installed the acetophenone functionality at the 5-position of
the quinoline frame followed by benzyl protection of the hydroxyl
group to give ether 67. Oxidation of quinoline 67 with mCPBA and
acylation of the resulting N-oxide with acetic anhydride and thermal
rearrangement produced quinolone 68. Bromination of the
methyl ketone and subsequent asymmetric reduction provided
(R)-alcohol 69. Bromohydrin 69 was then converted to the epoxide
using potassium carbonate prior to amination of the epoxide with
dihydroindene intermediate 65 to furnish the indacaterol skeleton
70. Hydrogenolytic debenzylation and maleate salt formation
provided indacaterol maleate (XI). 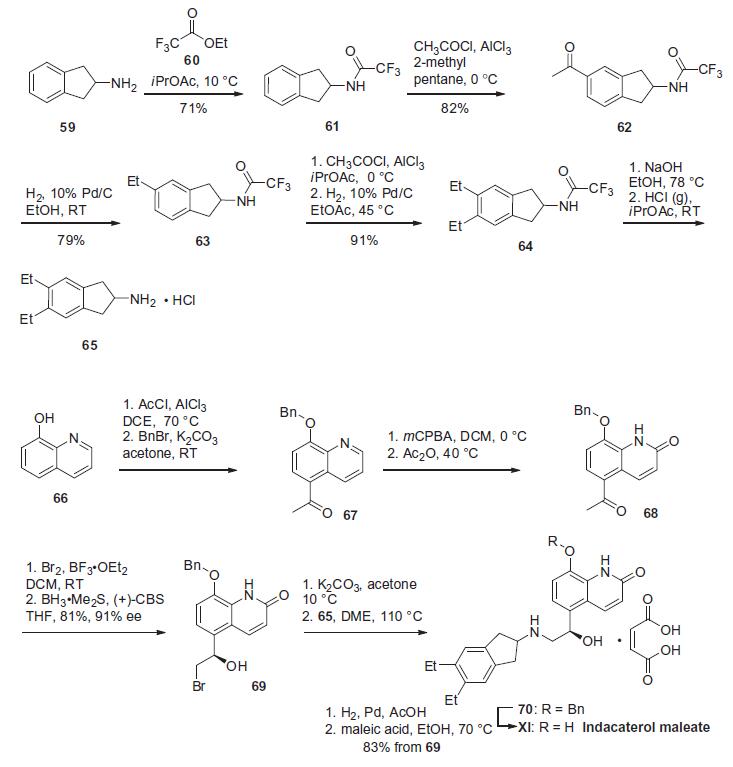
|
| | Indacaterol Maleate Preparation Products And Raw materials |
|