| Description | Benzamide appears as off-white crystals or powder. It is combustible and incompatible with strong oxidising agents and strong bases. On combustion and thermal decomposition, it emits nitrogen oxides, carbon monoxide, and carbon dioxide.
Benzamide is a carbonic acid amide of benzoic acid. Benzamide exhibits an angle of about 15º with the plane of the amide group; this shows that benzamide molecule is not flat. The rotation of the amide group relative to the aromatic ring may result from the repulsion interaction between the hydrogen atoms of the amide group and those of the aromatic ring.
|
| Chemical Properties | Benzamide is a combustible, colorless to beige, off-white, crystalline solid; freezing/melting point=132-133° C. It is slightly soluble in water, and soluble in many organic solvents.

Benzamide was used to study the mechanism of photocatalytic decomposition of aqueous solution of acetic acid, acetamide and acetonitrile in the presence of semiconductors. It was used to develop a robust screening method to study biotransformations using (+)-γ-lactamase enzyme.
|
| Uses | Organic synthesis.
Benzamide on radioiodination by different labeling procedures results in large-scale production of radioiodinated benzamides having potential therapeutic application for patients with metastatic malignant melanoma. |
| Uses | Benzamide is utilized to study the mechanism of photocatalytic decomposition of aqueous solution of acetic acid, acetamide and acetonitrile in the presence of semiconductors. It is used as a nictoinamide-mimic PARP inhibitor and neuroprotectant. Further, it is used to develop a robust screening method to study biotransformations using (+)-gamma-lactamase enzyme. It is also employed in the determination of glycine. In addition to this, it is used as an intermediate in organic synthesis as well as in the production of pharmaceuticals and dyes. |
| Preparation | Take a mixture of 5 ml concentrated ammonia and 5 ml water in a conical flask with a well-fitting cork. Add 2 ml (2.4 g.) benzoyl chloride, cork the flask and shake vigorously. Heat generates due to the reaction, hence hold the cork securely during shaking. After 15 min not even a trace of oily benzoyl chloride remains. Filter the fine flakes, wash with cold water and recrystallise from hot water: yield, 1-5 g. Colourless crystals of benzamide.
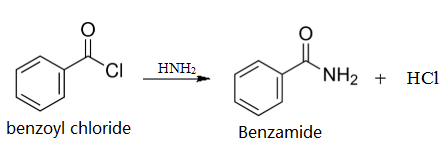
Preparation of benzamide from benzoyl chloride
|
| Definition | ChEBI: An aromatic amide that consists of benzene bearing a single carboxamido substituent. The parent of the class of benzamides. |
| Synthesis Reference(s) | The Journal of Organic Chemistry, 59, p. 4114, 1994 DOI: 10.1021/jo00094a021
Chemical and Pharmaceutical Bulletin, 39, p. 1152, 1991 DOI: 10.1248/cpb.39.1152
Synthetic Communications, 20, p. 1445, 1990 DOI: 10.1080/00397919008052860 |
| General Description | White powder. |
| Air & Water Reactions | Insoluble in water. |
| Reactivity Profile | Benzamide reacts with azo and diazo compounds to generate toxic gases. Forms flammable gases with strong reducing agents. Mixing with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. Combustion generates toxic mixed oxides of nitrogen (NOx). |
| Hazard | Depresses the central nervous system;
toxic.
|
| Fire Hazard | Flash point data for Benzamide are not available, however Benzamide is probably combustible. |
| Biochem/physiol Actions | Inhibits poly(ADP-ribose) polymerase (PARP). |
| Clinical Use | Benzamide on radioiodination by different labeling procedures results in large-scale production of radioiodinated benzamides having potential therapeutic application for patients with metastatic malignant melanoma. |
| Potential Exposure | Benzamide is used in organic
synthesis. |
| Purification Methods | Crystallise it from hot water (about 5mL/g), EtOH or 1,2-dichloroethane, and dry it in air. It has also been crystallised from dilute aqueous NH3, H2O, Me2CO, then *C6H6 using a Soxhlet extractor. Dry it in an oven at 110o for 8hours and store in a desiccator over 99% H2SO4. [Bates & Hobbs J Am Chem Soc 73 2151 1951, Beilstein 9 IV 725.] |




