| Chemical Properties | yellow to orange crystalline powder |
| Chemical Properties | Cadmium sulfide is an odorless, crystalline,
lemon yellow to orange solid. |
| Physical properties | Yellow to orange crystal; occurs as two polymorphs, hexagonal alpha form and cubic beta form; exhibits stable wurtzite structure at lower temperature, and zinc blende type structure at higher temperatures; the beta form converts to alpha form when heated at 750°C in sulfur atmosphere; sublimes at 980°C; practically insoluble in water (1.3 mg/L at 20°C); Ksp 3.6x10-29; dissolves in dilute mineral acids on heating or concentrated acids at ordinary temperatures (decomposes with liberation of H2S). |
| Occurrence | Cadmium sulfide occurs in nature as the mineral greenoktite. The compound is widely used in pigments for paints, baking enamels, ceramics and plastics. It imparts bright yellow to maroon, with strong retention of color and resistance to alkalis. It also is used in inks, phosphors, and fluorescent screens. Other applications of this compound are in photovoltaic and solar cells (for converting solar energy to electrical energy), photoconductors (in xerography), thin film transistors and diodes, rectifiers, scintillation counters, pyrotechnics, and smoke detectors. |
| Uses | cadmium sulfide is used as a colorant for paints and rubber; cadmium acetate is used in the production of craftware. |
| Uses | Used in the production of solar cells where it is used as a buffer layer in the manufacture of CIGS (Copper -Indium-Gallium-Selenide) solar cells |
| Uses | As a pigment being fast to light and not affected by H2S; color for soaps; coloring glass yellow; coloring textiles, paper, rubber; in printing inks, ceramic glazes, fireworks; in phosphors and fluorescent screens; in scintillation counters, semiconductors, photoconductors. |
| Definition | A native cadmium sulfide containing 77.7% cadmium. Ore of cadmium |
| Definition | Greenockite: a mineral form ofcadmium sulphide, CdS. |
| Production Methods | Cadmium sulfide may be prepared by the reaction between
hydrogen sulfide and cadmiumvapor at 800 Cor by heating a
mixture of cadmium or cadmium oxide with sulfur. |
| Preparation | Cadmium sulfide may be prepared by precipitation from an aqueous solution of its soluble salts such as cadmium chloride or cadmium nitrate by passing hydrogen sulfide. The reactions may be carried out in acidic, neutral or alkaline solutions using various cadmium salts to obtain different crystal modifications as shown in the table below.
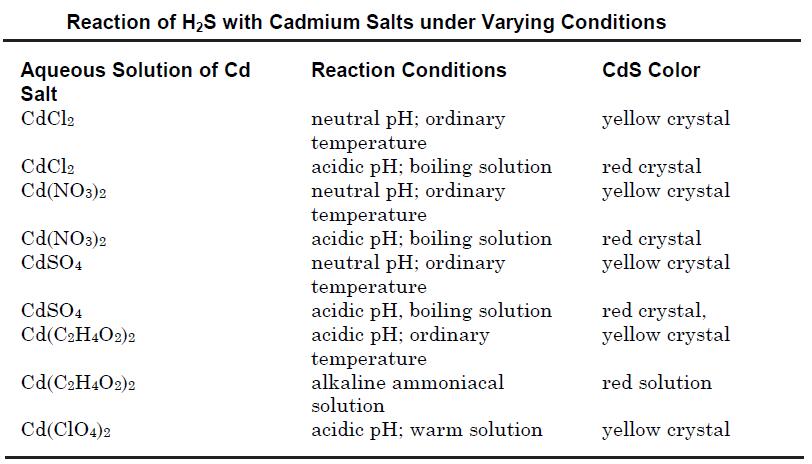
Cadmium sulfide also may be obtained by treatment of sodium or other alkali metal sulfide solution with that of a soluble cadmium salt. The compound also may be prepared by heating a mixture of cadmium or its oxide with sulfur at 800°C; or by the reaction of H2S with cadmium vapor at 800°C.
|
| General Description | Natural occurrence: hawleyite (structural type of sphalerite) and greenockite (structural type of wurtzite) |
| Hazard | A confirmed carcinogen, highly toxic. See
cadmium.
|
| Safety Profile | Confirmed human
carcinogen with experimental carcinogenic
and tumorigenic data. Moderately toxic by
ingestion and inhalation. Human mutation
data reported. When heated to
decomposition it emits very toxic fumes of
Cd and SOx. See also CADMIUM
COMPOUNDS and SULFIDES |
| Potential Exposure | Used in pigments; as an active ingredient in dandruff shampoos; making photoconductors, solar
cells, and other electronic components. |
| Shipping | UN2570 Cadmium compounds, Hazard Class:
6.1; Labels: 6.1-Poisonous materials, Technical Name
Required. |
| Incompatibilities | Contact with water or moisture releases
poisonous hydrogen sulfide gas. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause |
| Waste Disposal | Use a licensed professional
waste disposal service to dispose of this material. All federal, state, and local environmental regulations must be
observed. Consult with environmental regulatory agencies
for guidance on acceptable disposal practices. Generators
of waste containing this contaminant (≥100 kg/mo) must
conform to EPA regulations governing storage, transportation, treatment, and waste disposa |