|
| | Chlorothiazide Basic information |
| Product Name: | Chlorothiazide | | Synonyms: | 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 6-chloro-, 1,1-dioxide;2h-1,2,4-benzothiadiazine-7-sulfonamide,6-chloro-,1,1-dioxide;Aldoclor;Alurene;Chloriazid;Chlorosal;Chlorothiazid;Chlorthiazide | | CAS: | 58-94-6 | | MF: | C7H6ClN3O4S2 | | MW: | 295.72 | | EINECS: | 200-404-9 | | Product Categories: | DIURIL;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;Sulfur & Selenium Compounds;API | | Mol File: | 58-94-6.mol |  |
| | Chlorothiazide Chemical Properties |
| Melting point | 342-343°C | | Boiling point | 608.8±65.0 °C(Predicted) | | density | 1.7303 (rough estimate) | | refractive index | 1.6100 (estimate) | | storage temp. | 2-8°C | | solubility | DMSO (Slightly), Methanol (Very Slightly, Heated) | | pka | 6.85, 9.45(at 25℃) | | form | neat | | color | White to Off-White | | Water Solubility | <0.1 g/100 mL at 19.5 ºC | | Merck | 14,2168 | | BCS Class | 4 | | Stability: | Stable. Incompatible with strong oxidizing agents. | | LogP | -0.240 | | CAS DataBase Reference | 58-94-6(CAS DataBase Reference) | | NIST Chemistry Reference | Chlorothiazide(58-94-6) | | EPA Substance Registry System | Chlorothiazide (58-94-6) |
| | Chlorothiazide Usage And Synthesis |
| Description | Chlorothiazide is a first-in-class thiazide diuretic initially discovered from its ability to inhibit carbonic anhydrase in vitro. As an antihypertensive agent, this thiazide increases renal excretion of sodium, potassium, chloride, and bicarbonate ions by inhibiting tubular reabsorptive mechanisms. | | Chemical Properties | Off-White Solid | | Originator | Diuril,Merck Sharp and,US,1957 | | Uses | This drug exhibits strong diuretic action during both acidosis and alkalosis. It is used for
arterial hypertension, in edematous syndromes of various genesis, congestive effects in
cardiovascular insufficiency, nephrosis and nephritis, and toxicosis. It is especially recommended
for hypertonic illnesses. It lowers intraocular pressure in a number of cases. | | Uses | Diuretic; antihypertensive. Hydrochlorothiazide EP Impurity A. | | Definition | ChEBI: 4H-1,2,4-benzothiadiazine 1,1-dioxide in which the hydrogen at position is substituted by chlorine and that at position 7 is substituted by a sulfonamide group. A diuretic, it is used for treatment of oedema and hypertension. | | Manufacturing Process | (A) m-Chloroaniline (64 g, 0.5 mol) was added dropwise with stirring to 375
ml of chlorosulfonic acid in a 3-liter round bottom, 3-necked flask cooled in an
ice bath. Sodium chloride (350 g) was added portionwise over a period of 1 to2 hours and the mixture then heated gradually in an oil bath to 150°C. After 3
hours at 150° to 160°C, the flask was cooled thoroughly in an ice bath and
the contents treated with a liter of cold water. The product was extracted with
ether and the extract washed with water and dried over sodium sulfate.
After removal of ether on the steam bath, the residual 5-chloroaniline-2,4-
disulfonyl chloride, which may be crystallized from benzene-hexane MP 130°
to 132°C, was cooled in an ice bath and treated with 150 ml of 28%
ammonium hydroxide in a 2-liter Erlenmeyer flask. The mixture was heated
on the steam bath for 1 hour, cooled and the product collected on the filter,
washed with water and dried. Upon crystallization from dilute alcohol 5-
chloro-2,4-disulfamylaniline was obtained as colorless needles, MP 251° to
252°C.
(B) A solution of 88 g of 5-chloro-2,4-disulfamylaniline in 1.1 liters of 88%
formic acid was heated under reflux for 2 hours. After removal of 200 ml of
solvent by distillation, one liter of water was added and the product collected,
washed with water and dried. Crystallization from dilute alcohol afforded 6-
chloro-7-sulfamyl-1,2,4-benzothiadiazine-1,1-dioxide as colorless needles, MP
342.5° to 343°C, as described in US Patent 2,809,194. | | Brand name | Diuril (Ovation). | | Therapeutic Function | Diuretic; Antihypertensive | | General Description | Crystals; white powder. | | Air & Water Reactions | Insoluble in water. | | Reactivity Profile | Alkaline aqueous solutions will decompose on standing or heating . | | Fire Hazard | Flash point data for Chlorothiazide are not available; however, Chlorothiazide is probably combustible. | | Mechanism of action | The diuretic action of chlorothiazide, like other drugs of this series, is caused by reduced
absorption of sodium and chloride ions by the kidneys during their simultaneous, intense
excretion from the organism. | | Safety Profile | Moderately_toxic by
intraperitoneal and intravenous routes.
Mddly toxic by ingestion. Experimental
reproductive effects. Has been implicated in
aplastic anemia. When heated to
decomposition it emits very toxic fumes of
SOx NOx and Cl-. | | Synthesis | Chlorothiazide, 1,1-dioxide 6-chloro-2H-1,2,4-benzothiadiazin-7-sulfonamide
(21.3.3) is synthesized in the exact same manner, is all thiazide diuretics. 3-
Chloroaniline (or 3-trifluoromethylaniline) undergoes sulfoylchlorination by chlorosulfonic
acid, forming 4,6-sulfonochloride-3-chloroaniline (21.3.1), the reaction of which with ammonia
gives 4,6-sulfonylamido-3-chloroaniline (21.3.2). Heating this with formamide leads to
formation of chlorothiazide (21.3.3). 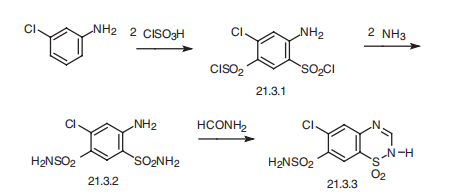
| | Veterinary Drugs and Treatments | In veterinary medicine, furosemide has largely supplanted the use
of thiazides as a general diuretic (edema treatment). Thiazides are
still used for the treatment of systemic hypertension,
nephrogenic
diabetes insipidus, and to help prevent the recurrence of calcium
oxalate uroliths in dogs.
Chlorothiazide is approved for use in dairy cattle for the treatment
of post parturient udder edema, but the veterinary labeled
product has been discontinued in the USA. |
| | Chlorothiazide Preparation Products And Raw materials |
|



