|
| | Fluorocytosine Chemical Properties |
| Melting point | 298-300 °C (dec.) (lit.) | | density | 1.3990 (estimate) | | vapor pressure | 0Pa at 25℃ | | storage temp. | 2-8°C | | solubility | Sparingly soluble in water, slightly soluble in ethanol (96 per cent) | | pka | 3.26(at 25℃) | | form | Crystalline Powder | | color | White to almost white | | Water Solubility | 1.5g/100mL (25 ºC) | | Sensitive | Light Sensitive | | Merck | 14,4125 | | BRN | 127285 | | BCS Class | 1 | | Stability: | Light Sensitive | | InChIKey | XRECTZIEBJDKEO-UHFFFAOYSA-N | | LogP | -1.36 at 22.1℃ and pH6.4-6.9 | | Dissociation constant | 2.74-10.71 at 21.4℃ | | CAS DataBase Reference | 2022-85-7(CAS DataBase Reference) |
| | Fluorocytosine Usage And Synthesis |
| Overview | A fluorinated pyrimidine, 5-flucytosine (fluorocytosine; 5-FC, Fig. 1), was initially developed as a potential anti-cancer agent but it was not sufficiently effective in the field of cancer chemotherapy[1]. Later, 5-FC proved to be active in experimental candidiasis and cryptococcosis in mice[2] and was used to treat human infections[3]. In addition to its activity against Candida and Cryptococcus, 5-FC also has an inhibitory activity against fungi causing chromoblastomycosis[4]; however, it is ineffective against infections caused by filamentous fungi. 5-FC has a high prevalence of primary resistance in many fungal species. Due to this primary resistance, 5-FC is used mainly in combination with other antifungals (primarily amphotericin B, AmB) and more recently it has been investigated in combination with other agents including fluconazole (FLU), ketoconazole (KTZ), itraconazole (ITRA), voriconazole (VORI) and echinocandins (e.g., micafungin, MICA and caspofungin, CAS). It is used only rarely as a single agent.
Flucytosine (5-FC) is a synthetic antimycotic compound, first synthesized in 1957. It has no intrinsic antifungal capacity, but after it has been taken up by susceptible fungal cells, it is converted into 5-fluorouracil (5-FU), which is further converted to metabolites that inhibit fungal RNA and DNA synthesis. Monotherapy with 5-FC is limited because of the frequent development of resistance. In combination with amphotericin B, 5-FC can be used to treat severe systemic mycoses, such as cryptococcosis, candidosis, chromoblastomycosis and aspergillosis.

Figure 1 the chemical structure of Fluorocytosine | | Mechanism of action and resistance | 5-FC is most active against yeasts, including Candida, Torulopsis and Cryptococcus spp., and against the dematiaceous fungi causing chromomycosis (Phialophora and Cladosporium spp.) and Aspergillus spp.[5] The MICs of 5-FC vary from 0.1 to 0.25 mg/L for these fungal species.
In Emmonsia crescens, Emmonsia parva, Madurella mycetomatis, Madurella grisea, Pyrenochaeta romeroi, Cephalosporium spp., Sporothrix schenckii and Blastomyces dermatitidis, MICs vary from 100 to 1000 mg/L.14 5-FC is also active against some protozoa, including Acanthamoeba culbertsoni both in vitro and in vivo and Leishmania spp. in patients.[5]
Antimycotic activity of 5-FC results from its rapid conversion into 5-fluorouracil (5-FU) by the enzyme cytosine deaminase, within susceptible fungal cells. There are two mechanisms involved by which 5-fluorouracil exerts its antifungal activity. The first mechanism includes the conversion of 5-fluorouracil through 5-fluorouridine monophosphate (FUMP) and 5-fluorouridine diphosphate (FUDP) into 5-fluorouridine triphosphate (FUTP)[6]. FUTP is further incorporated into fungal RNA in place of uridylic acid; this alters the aminoacylation of tRNA, disturbs the amino acid pool and inhibits protein synthesis[6]. The second mechanism is the metabolism of 5-FU into 5-fluorodeoxyuridine monophosphate (FdUMP) by uridine monophosphate pyrophosphorylase[6]. FdUMP is a potent inhibitor of thymidylate synthase, which is a key enzyme involved in DNA synthesis and nuclear division[7]. Thus, 5-FC acts by interfering with pyrimidine metabolism and protein synthesis in the fungal cell. These activity results in cell lysis and death.
The occurrence of resistance with the use of 5-FC has been widely described and precludes use of 5-FC as a single agent[8, 10] Two basic mechanisms of resistance can be distinguished: (i) certain mutations can result in a deficiency in the enzymes necessary for cellular transport and uptake of 5-FC or for its metabolism (i.e. cytosine permease, uridine monophosphate pyrophosphorylase or cytosine deaminase);[9,11] (ii) resistance may result from increased synthesis of pyrimidines, which compete with the fluorinated antimetabolites of 5-FC and thus diminish its antimycotic activity.[9] It has been shown that defective uridine monophosphate pyrophosphorylase is the most frequently occurring type of acquired 5-FC resistance in fungal cells.[12] Normark & Schönebeck have reported that two different phenotypes of 5-FC-resistant strains can be recognized:[10] strains of resistance phenotype class 1 are not affected by 5-FC at high concentrations (these are the totally (intrinsically) resistant strains), while those of class 2 are susceptible to 5-FC at low concentrations but, after long exposure to 5-FC (even at high concentrations) resistance develops (these are said to be partially resistant or to have acquired resistance). Development of resistance in the latter strains probably results from selection of non-susceptible mutants, leading to a secondary resistant population.[9]
The incidence of resistance to 5-FC varies between species.20 Up to 7–8% of intrinsically resistant strains are found among pretreatment isolates of C. albicans, unspeciated candida and Torulopsis glabrata. In C. neoformans the incidence of resistance is lower (1–2%), but in Candida spp. other than C. albicans it is 22%, because of the prevalence of generally less sensitive species such as Candida tropicalis and Candida krusei[13]. The exact incidence of primary 5-FC resistance is not clear. Different investigators report rates ranging between 8% and 44% for Candida spp[14]. Possible factors contributing to this wide range include the susceptibility methods used, local factors involving use of antifungal agents and differences in the prevalence of various Candida spp[14].
| | Pharmacokinetic and dosage | 5-FC is absorbed very rapidly and almost completely: 76–89% is bioavailable after oral administration.[16] In patients with normal renal function, peak concentrations are attained in serum and other body fluids within 1–2 h.[15, 16]. 5-FC penetrates well into most body sites, including cerebrospinal, vitreous and peritoneal fluids, and into inflamed joints, because it is small and highly water-soluble and is not bound by serum proteins to a great extent[15-17]. 5-FC is principally eliminated by the kidneys and the plasma clearance of the drug is closely related to creatinine clearance[15, 17]. 5-FC is only minimally metabolized in the liver. Renal elimination is via glomerular filtration; no tubular resorption or secretion takes place. The half-life of 5-FC is c.3–4 h in patients with normal renal function, but can be extended up to 85 h in patients with severe renal insufficiency.[12, 16, 18] Renal insufficiency alters 5-FC pharmacokinetics since it slows absorption, prolongs serum half-life and decreases clearance[15]. The apparent volume of distribution of 5-FC approaches that of total body water and is not altered by renal failure.
Dosage must be adjusted in patients with renal impairment. Various recommendations have been made[15-18]. Daneshmend & Warnock have suggested the following guidelines for the administration of 5-FC to patients with renal insufficiency.[15]. In patients with a creatinine clearance of >40 mL/min, a standard dose of 37.5 mg/kg every 6 h should be used. If the creatinine clearance is between 20 and 40 mL/min, the recommended dose is 37.5 mg/kg every 12 h. In patients with a creatinine clearance of <20 mL/ minute, the dose of 5-FC should be 37.5 mg/kg once daily. Finally, if the creatinine clearance is <10 mL/min, frequent determinations of 5-FC concentration should be used as guidance for the frequency of dosing.
| | Toxicity and side effects | 5-FC is known to have some relatively minor side effects, such as nausea, vomiting and diarrhoea, it also has more severe side effects, including hepatotoxicity and bonemarrow depression. Gastrointestinal side effects, the most common and least harmful side effects associated with 5-FC treatment, include nausea, diarrhoea and, occasionally, vomiting and diffuse abdominal pain. They occur in approximately 6% of patients treated with 5-FC[18]. Although these side effects are usually not severe; two cases of ulcerative colitis and bowel perforation have been reported[19]. Hepatotoxicity can occur during 5-FC treatment. In most cases it involves increases in serum concentrations of transaminases and alkaline phosphatase[20]. The incidence of hepatotoxicity is between 0 and 25%[20]. The most severe toxicity associated with 5-FC treatment are bone-marrow depression. There have been several reports of serious or life-threatening leucocytopenia, thrombocytopenia and/or pancytopenia[21-23]. The mechanism of toxicity of 5-FC is still not fully understood. It is likely that some of the side effects caused by 5-FC, for example hepatotoxicity and bone-marrow depression, are dose-dependent, although not all reports support this theory. Furthermore, it has been postulated that conversion of 5-FC to certain metabolites, especially 5-FU, could be one of the mechanisms of development of 5-FC-associated toxicity.
| | References |
- Heidelberg C, Chaudhuri NK, Danneberg P et al. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature 1957; 179(4561): 663–666
- Grunberg E, Titsworth E, Bennett M. Chemotherapeutic activity of 5-fluorocytosine. Antimicrob Agents Chemother 1963; 161:566–568
- Tassel D, Madoff MA. Treatment of Candida sepsis and Cryptococcus meningitis with 5-fluorocytosine. A new antifungal agent. JAMA 1968; 206(4): 830–832
- Benson JM, Nahata MC. Clinical use of systemic antifungal agents. Clin Pharm 1988; 7(6): 424–438
- Scholer, H. J. (1980). Flucytosine. In Antifungal Chemotherapy, (Speller, D. C. E., Ed.), pp. 35–106. Wiley, Chichester.
- Waldorf AR, Polak A. Mechanisms of action of 5-fluorocytosine. Antimicrob Agents Chemother 1983; 23(1):79–85
- Diasio RB, Bennett JE, Myers CE. Mode of action of 5-fluorocytosine. Biochem Pharmacol 1978; 27(5):703–707
- Polak, A. & Scholer, H. J. (1975). Mode of action of 5-fluorocytosine and mechanisms of resistance. Chemotherapy 21, 113–30.
- Polak, A. (1977). 5-Fluorocytosine—current status with special references to mode of action and drug resistance. Contributions to Microbiology and Immunology 4, 158–67.
- Normark, S. & Schönebeck, J. (1973). In vitro studies of 5-fluorocytosine resistance in Candida albicans and Torulopsis glabrata. Antimicrobial Agents and Chemotherapy 2, 114–21.
- Fasoli, M. & Kerridge, D. (1988). Isolation and characterization of fluoropyrimidine-resistant mutants in two Candida species. Annals of the New York Academy of Sciences 544, 260–3.
- Francis, P. & Walsh, T. J. (1992). Evolving role of flucytosine in immunocompromised patients: new insights into safety, pharmacokinetics, and antifungal therapy. Clinical Infectious Diseases 15, 1003–18.
- Medoff, G. & Kobayashi, G. S. (1980). Strategies in the treatment of systemic fungal infections. New England Journal of Medicine 302, 145–55.
- Armstrong, D. & Schmitt, H. J. (1990). Older drugs. In Chemotherapy for Fungal Diseases, (Ryley, J. F., Ed.), pp. 439–54. Springer-Verlag, Berlin.
- Daneshmend, T. K. & Warnock, D. W. (1983). Clinical pharmacokinetics of systemic antifungal drugs. Clinical Pharmacokinetics 8, 17–42.
- Cutler, R. E., Blair, A. D. & Kelly, M. R. (1978). Flucytosine kinetics in subjects with normal and impaired renal function. Clinical Pharmacology and Therapeutics 24, 333–42.
- Block, E. R., Bennett, J. E., Livoti, L. G., Klein, W. J., MacGregor, R. R. & Henderson, L. (1974). Flucytosine and amphotericin B: hemodialysis effects on the plasma concentration and clearance. Studies in man. Annals of Internal Medicine 80, 613–7.
- Schönebeck, J., Polak, A., Fernex, M. & Scholer, H. J. (1973). Pharmacokinetic studies on the oral antimycotic agent 5-fluorocytosine in individuals with normal and impaired kidney function. Chemotherapy 18, 321–36.
- Benson, J. M. & Nahata, M. C. (1988). Clinical use of systemic antifungal agents. Clinical Pharmacy 7, 424–38.
- Bennet, J. E. (1977). Flucytosine. Annals of Internal Medicine 86, 319–21.
- Kauffman, C. A. & Frame, P. T. (1977). Bone marrow toxicity associated with 5-fluorocytosine therapy. Antimicrobial Agents and Chemotherapy 11, 244–7.
- Schlegel, R. J., Bernier, G. M., Bellanti, J. A., Maybee, D. A., Osborne, G. B., Stewart, J. L. et al. (1970). Severe candidiasis associated with thymic dysplasia, IgA deficiency, and plasma antilymphocyte effects. Pediatrics 45, 926–36.
- Meyer, R. & Axelrod, J. L. (1974). Fatal aplastic anemia resulting from flucytosine. Journal of the American Medical Association 228, 1573.
| | Description | 5-Fluorocytosine (5-FC), a fluorinated pyrimidine analog, is a synthetic antimycotic prodrug that is converted by cytosine deaminase to 5-fluorouracil. 5-Fluorouracil, a widely used cytotoxic drug, is further metabolized to fluorinated ribo- and deoxyribonucleotides, resulting in the inhibition of DNA and protein synthesis, which has multiple effects including inhibition of Candida species and C. neoformans infections and cytotoxicity towards cancer cells. In combination with a retroviral replicating vector carrying a cytosine deaminase prodrug-activating gene, 5-FC has been shown to selectively eliminate CT26 and Tu-2449 tumor cells in vitro (IC50s = 4.2 and 1.5 μM, respectively) and to significantly improve survival and reduce tumor size (at a dose of 500 mg/kg) in two different syngeneic mouse glioma models. | | Chemical Properties | White Crystalline Solid | | Originator | Ancobon,Roche,US,1972 | | Uses | 5-FC is a toxic antifungal/antimicrobial agent | | Uses | 5-Fluorocytosine acts as an antidiabetic, antifungal and antimicrobial agent. It is useful for the treatment of serious infections arises due to susceptible strains of Candida or Cryptococcus neoformans and chromomycosis. Further, it is employed in studies on TMP biosynthesis. | | Definition |
ChEBI: Flucytosine is an organofluorine compound that is cytosine that is substituted at position 5 by a fluorine. A prodrug for the antifungal 5-fluorouracil, it is used for the treatment of systemic fungal infections. It has a role as a prodrug. It is an organofluorine compound, a pyrimidone, an aminopyrimidine, a nucleoside analogue and a pyrimidine antifungal drug. It is functionally related to a cytosine.
| | Indications | Flucytosine (Ancobon) is a synthetic, fluorinated pyrimidine that is structurally
related to fluorouracil (FU) and floxuridine. It can be fungistatic and
fungicidal. Although it is used more frequently in the treatment of systemic
infections caused by Candida and Cryptococcus, dermatologic indications
may include infections due to chromomycosis, sporotrichosis, Cladosporium,
and Sporothrix species. It is generally ineffective against Aspergillus species. | | Manufacturing Process | The preparation of 5-fluorouracil is given under "Fluorouracil." As described in
US Patent 3,040,026, 5-fluorouracil is then subjected to the following steps to
give flucytosine.
Step 1: 2,4-Dichloro-5-Fluoropyrimidine - A mixture of 104 grams (0.8 mol)
of 5-fluorouracil, 1,472 grams (9.6 mols) of phosphorus oxychloride and 166
grams (1.37 mols) of dimethylaniline was stirred under reflux for 2 hours.
After cooling to room temperature, phosphorus oxychloride was removed by
distillation at 18 to 22 mm and 22° to 37°C. The residue was then poured into
a vigorously stirred mixture of 500 ml of ether and 500 gram of ice. After
separating the ether layer, the aqueous layer was extracted with 500 ml, then
200 ml of ether. The combined ether fractions were dried over sodium sulfate,
filtered, and the ether removed by vacuum distillation at 10° to 22°C. The
residue, a yellow solid melting at 37° to 38°C, weighed 120 grams
corresponding to a 90% yield. Vacuum distillation of 115 grams of this
material at 74° to 80°C (16 mm) gave 108 grams of white solid melting at
38° to 39°C corresponding to an 84.5% yield.
Step 2: 2-Chloro-4-Amino-5-Fluoropyrimidine - To a solution of 10.0 grams
(0.06 mol) of 2,4-dichloro-5-fluoropyrimidine in 100 ml of ethanol, 25 ml of
concentrated aqueous ammonia were slowly added. A slightly opalescent
solution resulted. The temperature gradually rose to 35°C. The solution was
then cooled in ice to 18°C and thereafter remained below 30°C. After three
hours, a Volhard titration showed that 0.0545 mol of chlorine was present in
ionic form. Storage in a refrigerator overnight resulted in some crystallization
of ammonium chloride. A white sludge, resulting from the evaporation of the
reaction mixture at 40°C, was slurried with 75 ml of water, filtered and
washed free of chloride. After drying in vacuo, the product melted at 196.5°
to 197.5°C, yield 6.44 grams. Evaporation of the mother liquors yielded a
second crop of 0.38 gram, raising the total yield to 6.82 grams (79.3%).
Step 3: 5-Fluorocytosine - A slurry of 34.0 grams (0.231 mol) of 2-chloro-4-
amino-5-fluoropyrimidine in 231 ml of concentrated hydrochloric acid was
heated in a water bath at 93° to 95°C for 125 minutes. The reaction was
followed by means of ultraviolet spectrophotometry using the absorption at
245, 285, and 300 mμ as a guide. The absorption at 300 mμ rose to a
maximum after 120 minutes and then dropped slightly. The clear solution was
cooled to 25°C in an ice bath, then evaporated to dryness under vacuum at
40°C. After slurrying with water three times and reevaporating, the residue
was dissolved in 100 milliliters of water. To this solution, cooled in ice, 29 ml
of concentrated ammonia were added dropwise. The resulting precipitate was
filtered, washed free of chloride with water, then with alcohol and ether. After
drying in vacuo at 65°C, the product weighed 22.3 grams. An additional 6.35
grams was obtained by evaporation of the mother liquor, thus yielding a total
of 28.65 grams (96.0%). | | Brand name | Ancobon (Valeant). | | Therapeutic Function | Antifungal | | Antimicrobial activity | The spectrum of activity is restricted to Candida spp., Cryptococcus
spp. and some fungi causing chromoblastomycosis. | | Acquired resistance | About 2–3 of Candida spp. isolates (more in some centers) are
resistant before treatment starts, and resistance may develop
during treatment. The most common cause of resistance
appears to be loss of the enzyme uridine monophosphate
pyrophosphorylase. | | Pharmaceutical Applications | A synthetic fluorinated pyrimidine available for intravenous
infusion or oral administration. | | Biochem/physiol Actions | Nucleoside analog that has antifungal activities. 5-FC is deaminated by cytosine deaminase to product 5-fluorouracil, resulting in RNA miscoding. 5-Fluorocytosine inhibits DNA and RNA synthesis and interferes with ribosomal protein synthesis. | | Mechanism of action | Flucytosine (5-flucytosine, 5-FC; Ancoban) is a fluorinated pyrimidine analogue of cytosine that was originally synthesized for possible use as an antineoplastic agent. It is indicated only for the treatment of serious systemic infections caused by susceptible strains of Candida and Cryptococcus spp.The mechanism of action of 5-fluorocytosine (5-FC)has been studied in detail.The drug enters the fungal cell by active transport onATPases that normally transport pyrimidines. Once insidethe cell, 5-fluorocytosine is deaminated in a reaction catalyzedby cytosine deaminase to yield 5-fluorouracil(5-FU). 5-Fluorouracil is the active metabolite of the drug.5-Fluorouracil enters into pathways of both ribonucleotideand deoxyribonucleotide synthesis. The fluororibonucleotidetriphosphates are incorporated into RNA, causingfaulty RNA synthesis. This pathway causes cell death. Inthe deoxyribonucleotide series, 5-fluorodeoxyuridinemonophosphate (F-dUMP) binds to 5,10-methylenetetrahydrofolicacid, interrupting the one-carbon pool substratethat feeds thymidylate synthesis. Hence, DNA synthesisis blocked. | | Pharmacology | 5-FC is well absorbed orally, with greater than 90%
bioavailability. The serum half-life is 3 to 5 hours, with
serum levels peaking 4 to 6 hours after a single dose.The
drug is widely distributed in body fluids, with cerebrospinal
fluid levels 60 to 80% of serum levels.The drug
also penetrates well into urine, aqueous humor, and
bronchial secretions.Minimal serum protein binding allows
more than 90% of each dose to be excreted in the
urine; significant dosage reductions are required in the
presence of renal impairment. 5-FC can be removed by
both hemodialysis and peritoneal dialysis. 5-FC conversion
to toxic metabolites may occur in mammalian cells
to a limited extent, which accounts for 5-FC toxicity. | | Pharmacokinetics | Oral absorption: Complete
Cmax 25 mg/kg 6-hourly oral: 70–80 mg/L after 1–2 h
Plasma half-life: 3–6 h
Volume of distribution: 0.7–1 L/kg
Plasma protein binding c. 12%
Absorption is slower in persons with impaired renal function,
but peak concentrations are higher. Levels in the CSF
are around 75% of the simultaneous serum concentration.
More than 90% of a dose of flucytosine is excreted in the
urine in unchanged form. The serum half-life is much longer
in renal failure, necessitating modification of the dosage regimen:
for patients with a creatinine clearance below 40 mL/
min the dosage interval should be doubled to 12 h; in severe
renal failure the dosage interval should be further increased to
once daily or less, based on frequent serum drug concentration
measurements. | | Clinical Use | Flucytosine has significant antifungal activity against
C. albicans, other Candida spp., C. neoformans, and the
fungal organisms responsible for chromomycosis. Not
considered the drug of choice for these fungal infections,
5-FC does remain useful as part of combination
therapy for systemic candidiasis and cryptococcal
meningitis and as an alternative drug for chromomycosis.
When it is used as monotherapy, resistance and
clinical failure are common. Potential mechanisms for
drug resistance include decreased fungal cell membrane
permeability and reduced levels of fungal
cytosine deaminase. Combination therapy with amphotericin
B and flucytosine in the treatment of
cryptococcal meningitis and deep-seated Candida infections,
such as septic arthritis and meningitis, permits
reduced dosing of amphotericin B and prevents the
emergence of 5-FC resistance. When higher doses of
amphotericin B are used, combination therapy with
5-FC confers no additional clinical benefit except in
the treatment of Candida endophthalmitis, where tissue
penetration remains problematic. | | Clinical Use | Candidosis (in combination with amphotericin B or fluconazole)
Cryptococcosis (in combination with amphotericin B or fluconazole)
Monitoring of flucytosine concentrations is desirable in all
patients, and mandatory in those with renal impairment. | | Side effects | Nausea, vomiting, abdominal pain and diarrhea are common.
Serious side effects include myelosuppression and hepatic
toxicity; they occur more frequently when serum concentrations
exceed 100 mg/L.
The nephrotoxic effects of amphotericin B can result in
elevated blood concentrations of flucytosine, and levels of the
latter drug should be monitored when these compounds are
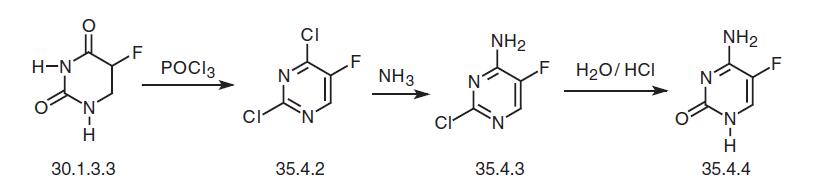
administered together. When 5-FC is prescribed alone to patients with normal renal function, skin rash, epigastric distress, diarrhea, and liver enzyme elevations can occur. | | Synthesis | Flucytosine, 5-fluorocytosine (35.4.4), is synthesized from fluorouracil (30.1.3.3). Fluorouracil is reacted with phosphorous oxychloride in dimethylaniline to make 2,4-dichloro-5-fluoropyrimidine (35.4.2), which is reacted with ammonia to make a product substituted with chlorine at the fourth position of the pyrimidine ring—4-amino- 2-chloro-5-fluoropyrimidine (35.4.3). Hydrolysis of the chlorovinyl fragment of this compound in a solution of hydrochloric acid gives the desired flucytosine.
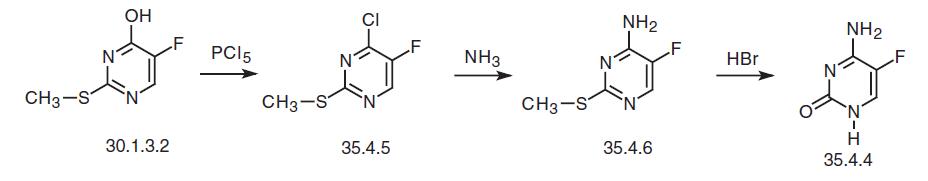
An alternative way of synthesis consists of making flucytosine from a precursor of fluorouracil—5-fluoro-2-methylthiouracil (30.1.3.2) using a somewhat analogous scheme. Treating 5-fluoro-2-methylthiouracil (30.1.3.2) with phosphorous pentachloride gives 4-chloro-5-fluoro-2-methylthiopyrimidine (35.4.5), which upon being reacted with ammonium is transformed into 4-amino-5-fluoro-2-methylthiopyrimidine (35.4.6). Hydrolysis of the methylthiovinyl fragment using concentrated hydrobromic acid gives the desired flucytosine.
 | | Drug interactions | Potentially hazardous interactions with other drugs
Cytarabine: concentration of flucytosine possibly
reduced. | | Metabolism | Flucytosine itself is not cytotoxic but, rather, is a pro-drug that is taken up by fungi and metabolized to 5-fluorouracil (5-FU) by fungal cytidine deaminase. Then, 5-FU is converted to 5-fluorodeoxyuridine, which as a thymidylate synthase inhibitor interferes with both protein and RNA biosynthesis. 5-Fluorouracil is cytotoxic and is employed in cancer chemotherapy. Human cells do not contain cytosine deaminase and, therefore, do not convert flucytosine to 5-FU. Some intestinal flora, however, do convert the drug to 5-FU, so human toxicity does result from this metabolism. |
| | Fluorocytosine Preparation Products And Raw materials |
|





