| Uses | Astemizole is a histamine H1-receptor antagonist with IC50 of 4.7 nM. Astemizole is also a potent inhibitor of ether à-go-go 1 (Eag1) and Eag-related gene (Erg) potassium channels. Astemizole has antineoplastic and antipruritic effects. |
| Description | Astemizole belongs to the second-generation class of non-sedating, non-anticholinergic
antihistamines. Its non-sedating properties appear to result from its
poor penetration of the blood brain barrier. As a result it shows no potentiation
of CNS depressants, including alcohol. Its long half-life allows once-daily dosing. |
| Chemical Properties | Crystalline Solid |
| Originator | Janssen (Belgium) |
| Uses | Astemizole is used for preventing and treating severe seasonal and chronic allergic rhinitis,
allergic conjunctivitis, hives, Quinke’s edema, other allergic conditions and dermatitis.
Synonyms of this drug are hismanal, histazol, and others. |
| Uses | scabicide |
| Uses | Nonsedating-type histamine H1-receptor antagonist. Potential for combination therapy with antivancer drugs such as doxorubicin in resistant leukemia. Antihistaminic |
| Definition | ChEBI: A piperidine compound having a 2-(4-methoxyphenyl)ethyl group at the 1-position and an N-[(4-fluorobenzyl)benzimidazol-2-yl]amino group at the 4-position. |
| Manufacturing Process | A mixture of 2.3 parts of 2-(4-methoxyphenyl)ethyl methanesulfonate, 4.9
parts of 1-[(4-fluorophenyl)methyl]-N-(4-piperidinyl)-1H-benzimidazol-2-
amine dihydrobromide, 3.2 parts of sodium carbonate, 0.1 part of potassium
iodide and 90 parts of N,N-dimethylformamide is stirred overnight at 70°C.
The reaction mixture is poured onto water. The product is extracted with
methylbenzene. The extract is washed with water, dried, filtered and
evaporated. The residue is purified by column-chromatography over silica gel
using a mixture of trichloromethane and methanol (98:2 by volume) as
eluent. The pure fractions are collected and the eluent is evaporated. The
residue is crystallized from 2,2'-oxybispropane, yielding 2.2 parts (48%) of 1-
(4-fluorophenylmethyl)-N-[1-[2-(4-methoxyphenyl)ethyl]-4-piperidinyl]-1Hbenzimidazol-
2-amine, MP 149.1°C. |
| Brand name | Hismanal (Janssen);Alermizol;Astezol;Astol;Histamanal;Novo-nastizol;EISMANAL. |
| Therapeutic Function | Antiallergic, Antihistaminic |
| World Health Organization (WHO) | The first clinically interesting histamine ti-antagonists were
introduced in the late forties and early fifties. Several histamine ti-antagonists have
a similar cardiac effect to that seen with astemizole and terfenadine. Serious
cardiovascular adverse reactions have been reported when used concomitantly with
imidazole antifungals and macrolide antibiotics. |
| Biological Activity | Orally active, potent histamine H 1 antagonist (IC 50 = 4 nM) that displays 20-fold, > 250-fold and > 250-fold selectivity over 5-HT, dopamine and muscarinic acetylcholine receptors respectively. Exhibits antimalarial activity in multidrug resistant strains in vitro (IC 50 = 227 - 734 nM). Also potent hERG K + channel blocker (IC 50 = 0.9 nM) that displays cardiotoxicity in vivo . |
| Biochem/physiol Actions | Astermizole is a potent hERG potassium channel blocker (IC50 of 0.9 nM) and may used as a pharmacological chaperone to correct folding defects and restore protein function for some mutated forms of hERG channels. It has also been studied for treatment of malaria, hERG and hEAG channel function in cancer and as a second generation antihistamine H-1 antagonist. |
| Safety Profile | Poison by subcutaneous andintravenous routes. Moderately toxic by ingestion. Humansystemic effects by ingestion: arrhythmias, coma, nauseaor vomiting, somnolence. When heated to decompositionit emits toxic fumes of F?? and NOx. |
| Synthesis | Astemizole, 1-[(4-fluorophenyl)methyl]-N-[1-[2-(4-methoxyphenyl) ethyl]-4-
piperidinyl]-benzimidazol-2-amine (16.1.31), is synthesized in a multi-stage synthesis from
1-carbethoxy-4-aminopiperidine and 2-nitroisothiocyanobenzol, from which a derivative of
thiourea (16.1.26) is synthesized upon their reaction. The nitro group of the product is
reduced and the further S-methoxided. In reaction conditions intermolecular cyclization into
a derivative of benimidazol, N-[1-[2-(4-carethoxy)]-4-piperidinyl]benzimidazol-2-amine
(16.1.28) occurs. The obtained aminobenzimidazole derivative is alkylated with 4-fluorobenzylchoride
into 1-[(flurophenyl)methyl]-N-[1-[2-(4-carethoxy)]-4-piperidinyl] benzimidazol-
2-amine (16.1.29). The carbethoxyl group of the resulting compound (16.1.29) is
hydrolyzed by hydrobromic acid, forming a non-substituted on the nitrogen atom derivative
of piperidine (16.1.30), the alkylation of which with 2-(4-methoxyphenyl)ethylmetanesulfonate
leads to the formation of astemizole (16.1.31). 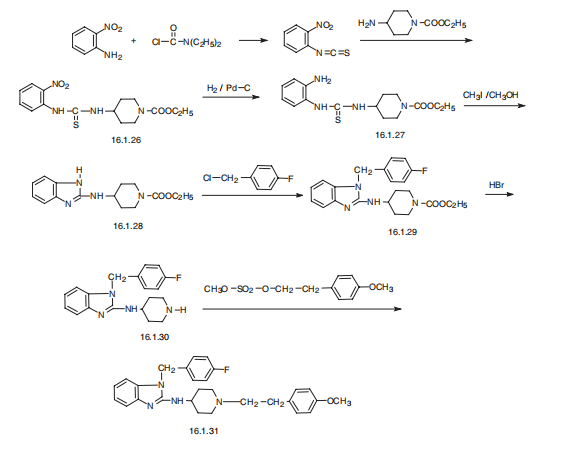
|
| storage | Store at +4°C |
| References | 1) Richards et al. (1984), Astemizole. A review of its pharmacodynamic properties and therapeutic efficacy; Drugs, 28 38
2) Laduron et al. (1982), In vitro and in vivo binding characteristics of a new long-acting histamine H1 antagonist, astemizole; Mol. Pharmacol., 21 294 |



