|
| | AMMONIUM CERIUM(IV) SULFATE Basic information |
| | AMMONIUM CERIUM(IV) SULFATE Chemical Properties |
| | AMMONIUM CERIUM(IV) SULFATE Usage And Synthesis |
| Uses | convenient reagent for oxidation of aromatic rings, and halophenols to quinones, for regioselective Baeyer–Villiger oxidation, and oxidative aromatization. | | Reactions | Cerium(IV) ion is a potent one-electron oxidant. cerium(IV) ammonium nitrate (CAN), is the most widely utilized cerium(IV) oxidizing agent, but cerium(IV) ammonium sulfate (CAS) is a good substitute when complications due to the involvement of nitrate ligands occur, resulting in side products such as nitrate esters.
Synthesis of Quinones by Oxidation of Aromatic Rings.
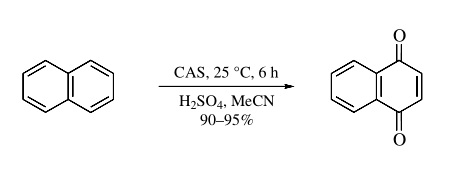
The most important application of CAS is in the oxidation of aromatic rings. CAN oxidizes polycyclic aromatic hydrocarbons only in moderate yields (20–60%), and these reactions are often complicated by the formation of nitrate esters. In contrast, CAS generally oxidizes aromatic hydrocarbons to quinones in goodyields.Forexample,naphthalene is oxidized to 1,4-naphthoquinone in excellent yield by CAS in a dilute mixture of H2SO4 and MeCN (eq1).
 | | storage | cerium(IV) ammonium sulfate is a stable reagent and precautions required for handling strong oxidizing agents such as potassium permanganate will be sufficient. Cerium is reputed to be of low toxicity. |
| | AMMONIUM CERIUM(IV) SULFATE Preparation Products And Raw materials |
|