|
| | Undecanedioic acid Chemical Properties |
| Melting point | 108-110 °C (lit.) | | Boiling point | 389.33°C (rough estimate) | | density | 1.1629 (rough estimate) | | vapor pressure | 10Pa at 20℃ | | refractive index | 1.4421 (estimate) | | storage temp. | Sealed in dry,Room Temperature | | solubility | methanol: 10 mg/mL, clear | | pka | 4.48±0.10(Predicted) | | form | Solid | | color | White to Off-White | | Water Solubility | 5.10g/L(21 ºC) | | BRN | 1780537 | | InChIKey | LWBHHRRTOZQPDM-UHFFFAOYSA-N | | LogP | 2.8 at 25℃ | | CAS DataBase Reference | 1852-04-6(CAS DataBase Reference) | | NIST Chemistry Reference | Undecanedioic acid(1852-04-6) | | EPA Substance Registry System | Undecanedioic acid (1852-04-6) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 | | HS Code | 29171900 |
| | Undecanedioic acid Usage And Synthesis |
| Description | Undecanedioic acid, in the form of a white powder or flake, is dibasic acids which is a family of organic compounds, also known as ‘long-chain dicarboxylic acids’. Undecanedioic acid is an alpha, omega-dicarboxylic acid that is nonane with two carboxylic acid groups at positions C-1 and C-9. It has a role as a metabolite. It is a conjugate acid of an undecanedioic acid anion. It has recommended applications in corrosion inhibitors, hot melt adhesives, high performance polyamides/nylon, metal-working fluid, lubircant, adhesive, powder coating, and more.
Undecanedioic acid has been found in parts of human aortas with advanced atherosclerotic lesions associated with intercellular matrix macromolecules and specifically with elastin, and may be the result of an increased hydrolysis of esters and (or) a decreased esterification. Undecanedioic acid has been found (among other unusual dicarboxylic acids) in the urine from patients under hopantenate therapy during episodes of Reye's-like syndrome.
| | Chemical Properties | white to light yellow powder | | Uses | Undecanedioic Acid is a bioactive compound found in Polygala tenuifolia root which is used as a functional food due to its attractive health benefits. | | Definition | ChEBI: An alpha,omega-dicarboxylic acid that is nonane with two carboxylic acid groups at positions C-1 and C-9. | | Flammability and Explosibility | Nonflammable | | Synthesis | Undecanedioic acid is obtained by reacting α -nitro ketone and NaOH in MeOH.To a solution of α -nitro ketone (0.7 mmol) in MeOH (4.2 ml), 25 ml of 0.5 M Na2HPO4 in a 1 N of NaOH were added and the resulting mixture was heated at 70 °C for 1-4 h, then Oxone (1.75 mmol) in water (3 ml) was added to the cold solution (r. t.). After 4 h the mixture was diluted with brine (10 ml), acidified to pH=2 with a 10% solution of HCl and extracted with EtOAc (3 x 20 ml). The combined organic layers were washed with brine (10 ml), dried over Na2SO4 and evaporated to yield pure dicarboxylic acid.
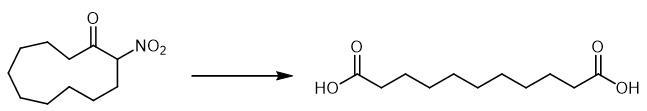
Fig The synthetic method 2 of Undecanedioic acid. |
| | Undecanedioic acid Preparation Products And Raw materials |
|