|
| | Poly(tetrahydrofuran) Basic information |
| | Poly(tetrahydrofuran) Chemical Properties |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 1 | | RTECS | MD0916000 | | HS Code | 3907 20 20 | | Toxicity | LD50 orally in Rabbit: > 5000 mg/kg |
| | Poly(tetrahydrofuran) Usage And Synthesis |
| Chemical Properties | mp 11 - 38 °C 100 - 4,400 mPa.s @ 40 °C | | Definition | ChEBI: A macromolecule composed of repeating oxybutylene units. | | Preparation | Low molecular weight polymers of tetrahydrofuran were introduced commercially in 1955. The polymers were intended for use in the preparation of
flexible polyurethane foams, being terminated by hydroxyl groups:
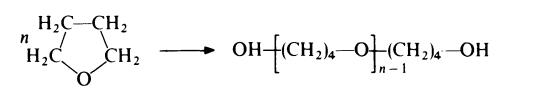
Although these polyethers produced good foams they were rather expensive
and were soon displaced by the cheaper propylene oxide-based polyethers.
However, these polymers of tetrahydrofuran (commonly called poly(oxytetramethylene)glycol) now find use in the preparation of polyamide, polyester and polyurethane thermoplastic elastomers.
Details of the manufacture of polytetrahydrofuran have not been disclosed.
Polymerization of tetrahydrofuran can be effected only by cationic initiators.
Combinations of metal halides with water are not effective but protic acids
(e.g. HCl04) and oxonium salts (e.g. (C2H5£?3O+BF4-) are efficient initiators.
Polymerization occurs by a ring-opening mechanism involving a tertiary
oxonium ion, e.g.
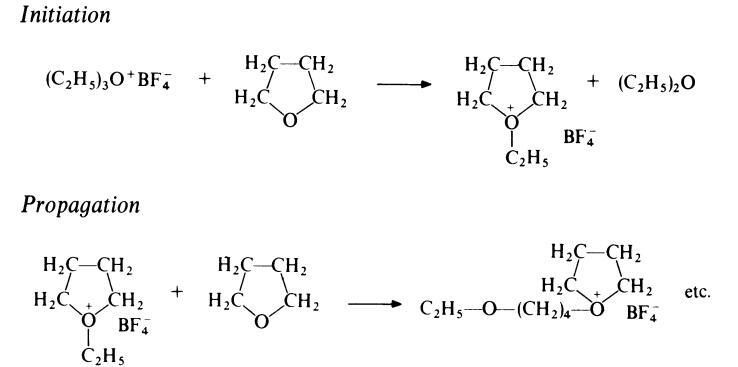
|
| | Poly(tetrahydrofuran) Preparation Products And Raw materials |
|