|
| | Phenanthrenequinone Chemical Properties |
| Melting point | 209-212 °C(lit.) | | Boiling point | 360 °C | | density | 1.405 | | refractive index | 1.5681 (estimate) | | Fp | 245 °C | | storage temp. | Store below +30°C. | | solubility | 7.5mg/l | | form | Powder | | color | Orange-brownish | | Water Solubility | Insoluble in water. | | Merck | 14,7213 | | BRN | 608838 | | Stability: | Stable. Incompatible with strong oxidizing agents. | | CAS DataBase Reference | 84-11-7(CAS DataBase Reference) | | NIST Chemistry Reference | 9,10-Phenanthroquinone(84-11-7) | | EPA Substance Registry System | 9,10-Phenanthrenedione (84-11-7) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36-24/25-22 | | RIDADR | UN3077 | | WGK Germany | 3 | | RTECS | SF7875000 | | Autoignition Temperature | 630 °C DIN 51794 | | TSCA | Yes | | HazardClass | 9 | | PackingGroup | III | | HS Code | 29146990 | | Hazardous Substances Data | 84-11-7(Hazardous Substances Data) | | Toxicity | LD50 orally in Rabbit: > 16000 mg/kg |
| | Phenanthrenequinone Usage And Synthesis |
| Description | 9,10-phenanthrenequinone (9,10-PQ) is a quinone molecule found in air pollution abundantly in the diesel exhaust particles (DEP). This compound has studied extensively and has been shown to develop cytotoxic effects both in vitro and in vivo. 9, 10-PQ has been proposed to play a critical role in the development of cytotoxicity via generation of reactive oxygen species (ROS) through redox cycling. This compound also reduces expression of glutathione (GSH), which is critical in Phase II detoxification reactions. | | Uses | 9,10-Phenanthrenequinon may be used for high quality passivation on silicon (100) surfaces. Quinones may serve as substrates for a variety of flavoenzymes.
The quinones of polycyclic aromatic hydrocarbons are present in abundance in all burnt organic material. On being used to passivate silicon surfaces, it reacts with the dangling bonds on the surface via a heteroatomic Diels-Alder reaction. On account of the Π-electron conjugation, the semi conducting nature of the silicon is unaffected.
| | Preparation | Phenanthrenequinone is obtained by the oxidation of the hydrocarbon phenanthrene C14H10. Upon reduction with sulphur dioxide, it yields phenanthrenehydroquinone which absorbs oxygen from the air forming a black quinhydrone. Upon further oxidation the phenanthrenequinone is again formed.
9,10-Phenanthrenequinone is prepared by oxidation of phenanthrene with dihydroxy phenylselenonium benzenesulfonate in boiling dioxane-water. 9-methoxyphenanthrene is obtained when the reaction is carried out in methanol.
https://www.tandfonline.com/doi/abs/10.1080/00397919708004809
| | Reactions | 9,10-Phenanthrenequinone (PQ) reacts with ketones under FeCl3 catalysis to furnish a variety of structurally diverse furan annulated products. While the reaction of PQ with acetone and cyclopentanone furnishes furan annulated ketals, its reaction with ethyl alkyl ketones provides 3-furaldehyde annulated products. In contrast to the reaction of PQ with cyclopentanone, its reaction with cyclohexanone furnishes a tetrahydrobenzofuran annulated secondary alcohol. The reactions of PQ with cycloheptanone and cyclooctanone take a different course to provide 7,8-dihydro-6H-cyclohepta[b]furan and 6,7,8,9-tetrahydrocycloocta[b]furan annulated products, respectively. Mechanistically, the above reactions go through aldol condensation, dehydration and cyclization to form furan-phenanthrene annulated products where in each step FeCl3 catalysis is involved.
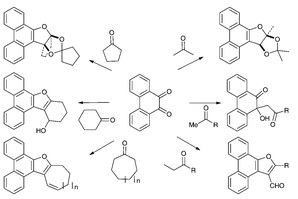
https://pubs.rsc.org/en/content/articlelanding/2012/ra/c2ra20499a
| | Chemical Properties | burnt-orange powder | | Uses | 9,10-Phenanthrenequinone is used in the formation of spirophosphoranes containing acyclic 5-methyl-2-phenyl-2H-1,2,3-diazaphosphol-4-yl substituent. | | Uses | 9,10-Phenanthrenequinon may be used for high quality passivation on silicon (100) surfaces. Quinones may serve as substrates for a variety of flavoenzymes. | | Definition | ChEBI: 9,10-phenanthroquinone is a member of phenanthrenes. | | Synthesis Reference(s) | The Journal of Organic Chemistry, 52, p. 3472, 1987 DOI: 10.1021/jo00391a065 | | General Description | The quinones of polycyclic aromatic hydrocarbons are present in abundance in all burnt organic material. On being used to passivate silicon surfaces, it reacts with the dangling bonds on the surface via a heteroatomic Diels-Alder reaction. On account of the Π-electron conjugation, the semi conducting nature of the silicon is unaffected. | | Safety Profile | Poison by acute intraperitoneal route. Questionable carcinogen with experimental tumorigenic data by skin contact. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes. | | Purification Methods | Crystallise the quinone from dioxane or 95% EtOH and dry it under vacuum. [Beilstein 7 IV 2565.] |
| | Phenanthrenequinone Preparation Products And Raw materials |
| Raw materials | Chromium(VI) oxide-->Sodium dichromate dihydrate-->Phenanthrene | | Preparation Products | 9-PHENANTHROL-->9-Hydroxy-9-fluorenecarboxylic acid-->FLURENOL-METHYL ESTER-->6H-dibenzo-(b,d)-pyran-6-one-->9,10-DICHLOROANTHRACENE-->3,6-Dibromo-phenanthrenequinone-->9-Carboxyfluorene-->10-(2',4'-DINITROPHENYLAZO)-9-PHENANTHROL-->7-METHYL-1,2,3,4-DIBENZOPHENAZINE�-->Phenanthro[9,10-g]pteridin-13(10H)-one, 11,12-dihydro-11-thioxo- |
|



