| Description | Nitrogen mustard (HN) was developed in three formulations: HN-1, HN-2, and HN-3. HN-1 was the first to be produced in the late 1920s and early 1930s. Originally, it was developed as a pharmaceutical and used to remove warts before it became a military agent. Agent H-2 was developed as a military agent and became a pharmaceutical. HN-3 was designed as a military mustard agent and is the only one that remains in military use. Therefore, this section will only cover the characteristics of HN-3 mustard agent. HN-3 is colorless to pale yellow with a butter-almond odor. The chemical formula for nitrogen mustard agent HN-3 is N(CH2CH2Cl)3. It will otherwise be ineffective against stopping the damage to the body. |
| Chemical Properties | White Solid |
| Chemical Properties | Highly toxic white to yellowish crystalline
solid or powder. May be available as an unstable aqueous
solution. Fish-like odor. |
| Uses | xanthine oxidase/dehydrogenase inhibitor |
| Uses | It has been used as an antineoplastic. A nitrogen mustard prepared by action of thionyl chloride on 2,2’(methylimino)-
diethanol in trichloroethylene. |
| Uses | Mechlorethamine hydrochloride USP (Mustargen)is used to treat Hodgkin’s disease; non-Hodgkin’s lymphomas; lymphosarcoma; cancer of breast, ovary, lung; neoplastic effusion. |
| Definition | ChEBI: The hydrochloride salt of mechlorethamine. |
| Indications | Mechlorethamine (Mustargen) is a cytotoxic alkylating
agent. Topical application of freshly prepared aqueous
solutions are used in patients with early stages of cutaneous
T-cell lymphoma. A major disadvantage to the
use of this drug is the rapid induction of allergic contact
dermatitis in some patients. |
| Indications | Mechlorethamine (nitrogen mustard; Mustargen), a derivative
of the war gas sulfur mustard, is considered to
be the first modern anticancer drug. In the early 1940s it
was discovered to be effective in the treatment of human
lymphomas. |
| Brand name | Mustargen (Ovation). |
| Biological Functions | Mechlorethamine is still used in regimens for cancers of the blood (e.g., Hodgkin's disease, chronic myelocytic, or chronic lymphocytic leukemia); fortunately, however, safer and still highly potent antineoplastic agents are now available. |
| General Description | White to off-white crystals or powder with a fishy odor. Initial pH (2% aqueous solution) 3.0-4.0. |
| General Description | Mechlorethamine is available in 10-mg vials for intravenous(IV) administration in the treatment of Hodgkin’slymphoma. It is part of the MOPP regimen used in treatingthis condition, which is comprised of mechlorethamine,vincristine (Oncovin), procarbazine, and prednisone. Theagent is also used topically in the treatment of mycosis fungoides,a rare type of cancer but the most common type ofcutaneous T-cell lymphoma. Additional uses have includedtreatment of cancers that have resulted in pleural effusion.Although the compound is a potent alkylating agent, resistancemay develop as a result of increased inactivation bysulfhydryl containing proteins such as glutathione andincreased expression of DNA repair mechanisms. Adverseeffects include dose-limiting myelosuppression and nausea/vomiting. There is a significant risk of extravasationupon IV administration, and the agent may produce painat the injection site. Additional adverse effects include alopecia, azoospermia, amenorrhea, hyperuricemia, and anincreased risk of secondary cancers. |
| Air & Water Reactions | Hygroscopic. Water soluble. |
| Reactivity Profile | Dry crystals are stable at temperatures up to 104° F. Chlormethine hydrochloride is incompatible with strong oxidizing agents. . |
| Hazard | Highly toxic, vesicant, and strongly irritant
to mucous membranes. |
| Fire Hazard | Flash point data for Chlormethine hydrochloride are not available. Chlormethine hydrochloride is probably combustible. |
| Mechanism of action | Mechlorethamine in aqueous solution loses a chloride
atom and forms a cyclic ethylenimmonium ion.This
carbonium ion interacts with nucleophilic groups, such
as the N7 and O6 of guanine, and leads to an interstrand
cross-linking of DNA. Although there is great variation
among normal and tumor tissues in their sensitivity to
mechlorethamine, the drug is generally more toxic to
proliferating cells than to resting or plateau cells.
Mechlorethamine has a chemical and biological half-life
in plasma of less than 10 minutes after intravenous injection.
Little or no intact drug is excreted in urine.
The major indication for mechlorethamine is
Hodgkin’s disease; the drug is given in the MOPP regimen. Other less reactive nitrogen
mustards are now preferred for the treatment of non-
Hodgkin’s lymphomas, leukemias, and various solid
tumors. |
| Clinical Use | Mechlorethamine is the only aliphatic nitrogen mustard currently on the U.S. market. Its use is limited by extremely high reactivity, which leads to rapid and nonspecific alkylation of cellular nucleophiles and excessive toxicity. It is a severe vesicant, and if accidental skin contact occurs, the drug must be inactivated with 2% sodium thiosulfate (Na2S2O3) solution. |
| Side effects | The dose-limiting toxicity of mechlorethamine is
myelosuppression; maximal leukopenia and thrombocytopenia
occur 10 to 14 days after drug administration,
and recovery is generally complete at 21 to 28 days.
Lymphopenia and immunosuppression may lead to activation
of latent herpes zoster infections, especially in
patients with lymphomas. Mechlorethamine will affect
rapidly proliferating normal tissues and cause alopecia,
diarrhea, and oral ulcerations. Nausea and vomiting may
occur 1 to 2 hours after injection and can last up to 24
hours. Since mechlorethamine is a potent blistering
agent, care should be taken to avoid extravasation into
subcutaneous tissues or even spillage onto the skin.
Reproductive toxicity includes amenorrhea and inhibition
of oogenesis and spermatogenesis. About half of
premenopausal women and almost all men treated for 6
months with MOPP chemotherapy become permanently
infertile. The drug is teratogenic and carcinogenic
in experimental animals. |
| Safety Profile | Confirmed carcinogen withexperimental carcinogenic, neoplastigenic, andtumorigenic data. Deadly poison by ingestion,intravenous, subcutaneous, intraperitoneal, and parenteralroutes. Experimental teratogenic and reproductive effects.Human systemic eff |
| Synthesis | Mechlorethamine, bis-(2-chloroethyl)methylamine (30.2.1.2), is made
by reacting methylamine with ethylene oxide, forming bis-(2-hydroxyethyl)methylamine
(30.2.1.1), which upon reaction with thionyl chloride turns into the desired mechlorethamine. 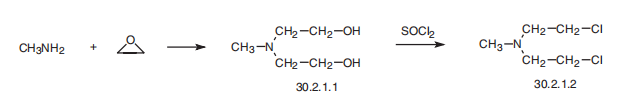
|
| Carcinogenicity | Nitrogen mustard hydrochloride is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals. In the literature, the names
“nitrogen mustard” and “nitrogen mustard hydrochloride” are used interchangeably. Only nitrogen mustard hydrochloride is produced commercially, so it is assumed that nitrogen mustard hydrochloride was used in all cancer studies in animals reported below. |
| Shipping | UN2928 Toxic solids, corrosive, organic, n.o.s.,
Hazard Class: 6.1; Labels: 6.1-Poisonous materials,
8-Corrosive material, Technical Name Required. UN2811
Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels:
6.1-Poisonous materials, Technical Name Required. |
| Waste Disposal | It is not appropriate to dispose
of expired or waste product such as lab chemicals by
flushing them down the toilet or discarding them to the
trash. Larger quantities shall carefully take into consideration
applicable EPA, and FDA regulations. If possible
return the lab chemicals to the manufacturer for proper disposal
being careful to properly label and securely package
the material. Alternatively, the waste lab chemicals shall be
labeled, securely packaged and transported by a state
licensed medical waste contractor to dispose by burial in a
licensed hazardous or toxic waste landfill or incinerator. |



