|
| Product Name: | Ozenoxacin | | Synonyms: | Ozenoxacin;T 3912;:3-Quinolinecarboxylic acid, 1-cyclopropyl-1,4-dihydro-8-methyl-7-[5-methyl-6-(methylamino)-3-pyridinyl]-4-oxo-;Ozenoxacin(T 3912);1-cyclopropyl-8-methyl-7-[5-methyl-6-(methylamino)pyridin-3-yl]-4-oxoquinoline-3-carboxylic acid;Ozenoxacin , T 3912,UNII-V0LH498RFO;Ozenoxacin Impurity 1;organic materials Ozenoxacin | | CAS: | 245765-41-7 | | MF: | C21H21N3O3 | | MW: | 363.41 | | EINECS: | 105-745-2 | | Product Categories: | | | Mol File: | 245765-41-7.mol |  |
| | Ozenoxacin Chemical Properties |
| Melting point | >255°C (dec.) | | Boiling point | 573.5±50.0 °C(Predicted) | | density | 1.372±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C(protect from light) | | solubility | DMSO (Slightly) | | pka | 6.46±0.50(Predicted) | | form | Solid | | color | Off-White |
| | Ozenoxacin Usage And Synthesis |
| Description | Ozenoxacin is used to treat impetigo (a skin infection caused by bacteria) in adults and children 2 months of age and older. Ozenoxacin is in a class of medications called antibacterials. It works by killing and stopping the growth of bacteria on the skin.
To date, ozenoxacin has been used in trials studying the treatment of impetigo. As of December 11, 2017 the FDA approved Ferrer Internacional S.A.'s Xepi (ozenoxacin 1%) as a topically applied cream indicated for the treatment of impetigo caused by Staphylococccus aureus or Streptococcus pyogenes in adult and pediatric patients 2 months of age and older. | | Pharmacokinetics | Although the exposure response relationship for ozenoxacin after it has been applied topically has not yet been studied, a formal relationship is unlikely because systemic exposure of ozenoxacin following its topical application has been measured to be negligible .
| | Mechanism of action | Ozenoxacin is a quinolone antibiotic drug. And, like most quinolones, ozenoxacin predominately executes its mechanism of action by entering into bacterial cells and acting to inhibit the bacterial DNA replication enzymes DNA gyrase A and topoisomerase IV . As DNA gyrase A and topoisomerase IV are essential to bacterial DNA replication activities including supercoiling, supercoil relaxation, chromosomal condensation, chromosomal decatenation and more, their inhibition is the principal action of ozenoxacin's mechanism and it has been demonstrated to be bactericidal against S. aureus and S. pyogenes organisms.
| | Description | Ozenoxacin is a novel,
nonfluorinated quinolone antibiotic discovered by Toyama
Chemical Co. Ltd. and developed by Maruho Co. Ltd.
Ozenoxacin was approved by the PMDA of Japan in September
2015 for the treatment of acne and skin infections. Ozenoxacin
shows potent antibacterial activity against anaerobic and
aerobic, gram-positive and -negative bacteria, especially those implicated in superficial skin infections such as S. aureus,
Staphylococcus epidermidis, and Propionibacterium acnes. The
mechanism of action of ozenoxacin involves the drug’s affinity
for DNA gyrase and DNA topoisomerase IV and upon binding
triggers bacterial apoptosis. | | Uses | Ozenoxacin is a non-fluorinated topical quinolone. It exhibits antimicrobial activity against?propionibacteria and staphylococci. Ozenoxacin can be used to treat acne and superficial skin infections. | | Definition | ChEBI: Ozenoxacin is a member of quinolines. | | Synthesis | A U.S. patent filed by co-workers at Toyama describes the
only publicly disclosed synthetic approach to this drug.12 The
drug?ˉs assembly hinges upon a key Stille coupling between a
quinolonyl bromide and a stannylpyridine.
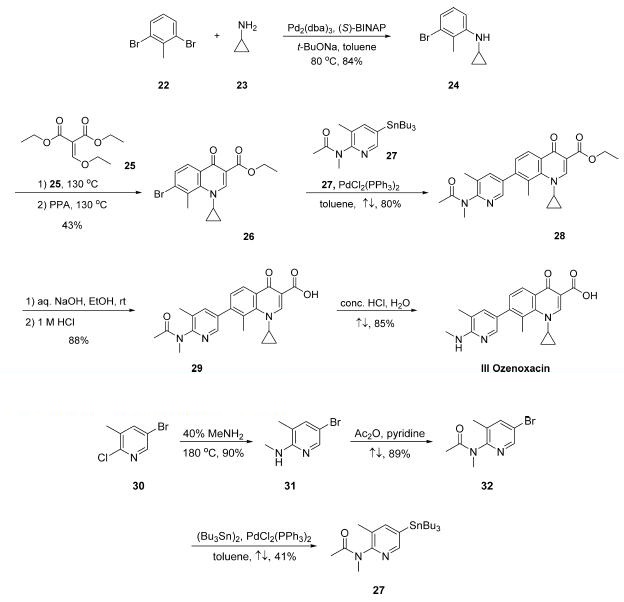
Buchwald-Hartwig coupling of commercially available 2,6-
dibromotoluene (22) and cyclopropylamine (23) gave Ncyclopropyl-
3-bromo-2-methylaniline 24 in 84% yield , and this step was followed by reaction with diethyl
ethoxymethylenemalonate (25) and subsequent cyclization
under acidic conditions to secure bromoquinoline 26 in 43%
yield over the two-step sequence. Stille coupling of 27 with
bromoquinoline 26 resulted in pyridyl quinoline adduct 28 in
80% yield. Saponification of ester 28 followed by acidic removal
of the N-acetyl group delivered the active pharmaceutical
ingredient ozenoxacin (III) in 75% yield.
The preparation of key stannane 27, which is not
commercially available and began
with the conversion of commercially available 5-bromo-2-
chloro-3-methylpyridine (30) to aminopyridine derivative 31
upon treatment with aqueous methylamine at elevated
temperature in a sealed vessel. The resulting aminopyridine
was subjected to acetic anhydride in pyridine, resulting in
acetamide 32 in good yield, and this coupling was followed by a
modest-yielding palladium-catalyzed installation of the stannyl
group to deliver subunit 27.
|
| | Ozenoxacin Preparation Products And Raw materials |
|