|
| Product Name: | Besifloxacin hydrochloride | | Synonyms: | (R)-7-(3-AMinohexahydro-1H-azepin-1-yl)-8-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-quinolin-3-carboxylic acid HCl;3-Quinolinecarboxylic acid, 7-[(3R)-3-aMinohexahydro-1H-azepin-1-yl]-8-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-, Monohydrochloride (9CI);(R)-7-(3-aMinoazepan-1-yl)-8-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid hydrochloride;Besifloxacin HCl;Unii-7506A6J57t;(R)-7-(3-Aminohexahydro-1H-azepin-1-yl)-8-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid hydrochloride;Besifloxacin hydrochloride;Becifloxacin Hydrochloride | | CAS: | 405165-61-9 | | MF: | C19H22Cl2FN3O3 | | MW: | 430.3 | | EINECS: | 696-612-6 | | Product Categories: | API;Inhibitors;Amines;Aromatics;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 405165-61-9.mol |  |
| | Besifloxacin hydrochloride Chemical Properties |
| Melting point | >210°C (dec.) | | storage temp. | Inert atmosphere,2-8°C | | solubility | Methanol (Slightly, Heated, Sonicated), Water (Slightly, Heated, Sonicated) | | form | Solid | | color | Off-White to Light Beige |
| | Besifloxacin hydrochloride Usage And Synthesis |
| Outline | Besifloxacin hydrochloride is developed by Bausch & Lomba as a new type of fluoroquinolones for the treatment of bacterial conjunctivitis (a non-viral caused infectious purulent eye irritation conjunctivitis ). Bacterial conjunctivitis which is a contagious eye disease, is a common disease that affects people of all ages. The clinical symptoms of infested eye including: foreign body sensation, burning sensation, tearing; conjunctival hyperemia, mucus or purulent discharge; eyelid edema, conjunctival edema and subconjunctival hemorrhage may occur in severe cases .
| | Besifloxacin hydrochloride ophthalmic suspension | Besifloxacin hydrochloride ophthalmic suspension (Besivance) is a fluoroquinolone drug recently approved for the topical treatment of bacterial conjunctivitis. This medicine can quickly kill common pathogens causing conjunctivitis , that is coagulase-negative staphylococci, Streptococcus pneumoniae, Staphylococcus aureus , Haemophilus influenzae bacteria, and other less common bacteria.. Besifloxacin is an effective drug to the Gram-positive and Gram-negative pathogens, include other fluoroquinolone-resistant bacteria , also it can balance the DNA gyrase and topoisomerase IV activity, slow the development of resistance. Topical administration can achieve stable and lasting high concentrations in human tears , and penetration in animal ocular tissue is good, showing excellent safety.
Pharmacokinetic and pharmacodynamic characteristics of Besifloxacin meet the criteria for successfully clearing most Gram-positive and Gram-negative bacteria, while exhibiting minimal systemic exposure.Besifloxacin biochemical properties, pharmacokinetics targeted to achieve/pharmacodynamic objectives and limited to local ophthalmic drug will slow the development of bacterial resistance, making Besifloxacin become attractive choice for acute bacterial conjunctivitis treatment.
The above information is edited by the chemicalbook of Tian Ye.
| | Description | Besifloxacin is a new fluoroquinolone antibacterial for ophthalmic
use. It is indicated for the treatment of bacterial conjunctivitis, one of
the most common ocular infections encountered in the primary care
setting. Although the previously marketed fluoroquinolones ciprofloxacin, levofloxacin, ofloxacin, gatifloxacin, and moxifloxacin have been
widely used to treat bacterial conjunctivitis, their continued utility is
hampered by the emergence of resistance among key ocular isolates
such as Staphylococcus aureus. Besifloxacin is the first
fluoroquinolone developed exclusively for topical ophthalmic use.
Unlike the previously marketed fluoroquinolones, besifloxacin has not
been used systemically and is not in development as a systemic agent. Its
exclusive indication as a topical agent is expected to reduce the overall
environmental exposure of bacteria to besifloxacin, which may contribute to a lower risk for the emergence of bacterial resistance. Fluoroquinolones derive their antibacterial activity via inhibition of two essential
bacterial enzymes, DNA gyrase and topoisomerase IV, which regulate
processes of DNA replication. Besifloxacin inhibits DNA gyrase and topoisomerase IV from Streptococcus pneumoniae (IC50 = 1 and 0.4 mg/ L, respectively) and Escherichia coli (IC50 = 1 and 10 mg/L, respectively). | | Chemical Properties | Pale Yellow Solid | | Originator | SSP Co. Ltd. (Japan) (Japan) | | Uses | A Fluoroquinolone antibiotic. | | Uses | Besifloxacin HCl is a fourth-generation fluoroquinolone antibiotic | | Brand name | Besivance | | Side effects | The most common adverse event reported in patients treated with besifloxacin ophthalmic suspension was conjunctival redness. Other less common adverse events were blurred vision, eye pain, eye irritation, eye pruritus, and headache. | | Synthesis | Besifloxacin is a fourth-generation fluoroquinolone antibiotic
which is marketed as besifloxacin hydrochloride. It was originally
developed by the Japanese firm SSP Co. Ltd and designated
SS734. SSP then licensed U.S. and European rights of SS734 for ophthalmic
use to InSite Vision, Inc., in 2000, who then developed an
eye drop formulation (ISV-403) and conducted preliminary clinical
trials before selling the product and all rights to Bausch & Lomb in
2003. The eye drop was approved by the United States Food and
Drug Administration (FDA) on May 29, 2009 and marketed under
the trade name Besivance. Besifloxacin has been found to inhibit
production of pro-inflammatory cytokines in vitro. The synthesis of
besifloxacin commences with commercially available ethyl
3-(3-chloro-2,4,5-trifluorophenyl)-3-oxopropanoate.Condensation of this ketoester with triethyl orthoformate
resulted in a mixture of vinylogous esters 14. Substitution with
cyclopropanamine converts 14 to the vinylogous amide 15 as an
unreported distribution of cis- and trans-isomers. This mixture
was treated with base at elevated temperature to give 16.
Presumably, the trans-isomer isomerizes to the cis-isomer, which
subsequently undergoes an intramolecular nucleophilic aromatic
substitution with concomitant saponification to construct
quinolone acid 16. Quinolone 16 is then subjected to another
nucleophilic substitution involving readily available iminoazepine
17 and the displacement reaction proceeds regioselectively to furnish
the atomic framework of besifloxacin (18). Acidic methanolysis
of 18 at elevated temperature gave besiflozacin (III). 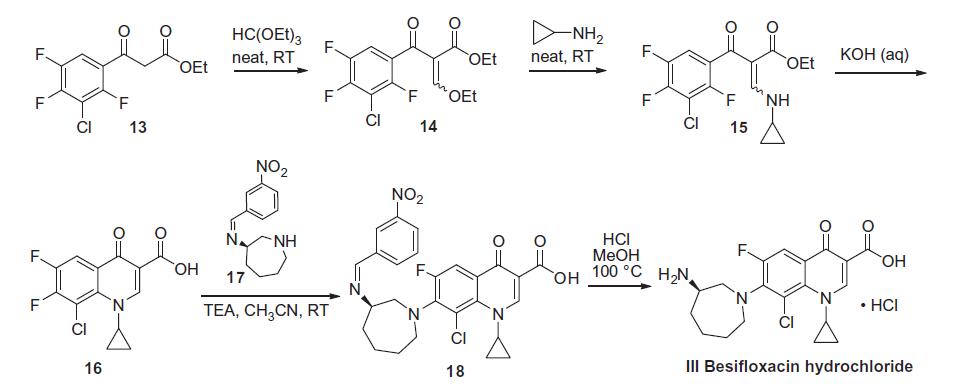
|
| | Besifloxacin hydrochloride Preparation Products And Raw materials |
|