|
| Product Name: | Tigecycline | | Synonyms: | (4s,4as,5ar,12as)-4,7-bis(dimethylamino)-9-[(tert-butylamino)acetamido]-3,10,12,12a-tetrahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracen-2-carboxamide;2-NAPHTHACENECARBOXAMIDE, 4,7-BIS(DIMETHYLAMINO)-9-[[[(1,1-DIMETHYLETHYL)AMINO]ACETYL]AMINO]-1,4,4A,5,5A,6,11,12A-OCTAHYDRO-3,10,12,12A-TETRAHYDROXY-1,11-DIOXO-, (4S,4AS,5AR,12AS)-;ecycL;Tig;tigecycline;TIGECYCLINE GLYCYLCYCLINE;(4S,4As,5aR,12as)-4,7-Bis(dimethylamino)-9-{(tert-butylamino)acetamido}-3,10,12,12a-octahydrotetracen-2-carboxamide;2-NAPHTHACENECARBOXAMIDE | | CAS: | 220620-09-7 | | MF: | C29H39N5O8 | | MW: | 585.65 | | EINECS: | 685-736-6 | | Product Categories: | pharmaceutical intermediate;Amines;API;Chiral Reagents;Intermediates & Fine Chemicals;Pharmaceuticals;Antibacterial;BDO;220620-09-7 | | Mol File: | 220620-09-7.mol |  |
| | Tigecycline Chemical Properties |
| Melting point | 164-166°C | | Boiling point | 890.9±65.0 °C(Predicted) | | density | 1.45±0.1 g/cm3(Predicted) | | storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C | | solubility | Soluble in DMSO (up to at least 25 mg/ml). | | form | Orange powder | | pka | 4.50±1.00(Predicted) | | color | Orange | | Merck | 14,9432 | | Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. | | CAS DataBase Reference | 220620-09-7(CAS DataBase Reference) |
| | Tigecycline Usage And Synthesis |
| Indications and Usage | Tigecycline is also called 9-tert-glycylaminomycetine or diclofenac, and it is a new type of venous injection antibiotic with broad-spectrum activities. It is a type of 9-tert-glycylaminomycetine derivative and is the first glycylcine antibiotic.
Tigecycline can serve as a second option after failed first-line treatment for multi-drug resistant bacteria, and it is also a new treatment option for patients who are allergic to penicillin or intolerable to other drugs. It can treat patients 18 years old or above with complex skin and skin structure infections or complex abdominal infections such as complex appendicitis, burn infections, abdominal abscesses, deep soft tissue infections, and ulcer infections.
| | Mechanisms of Action | Tigecycline’s mechanisms of action are similar to those of tetracycline antibiotics, which are binding with bacterial 30S ribosomes to prevent transfer RNA from entering, making it impossible for amino acids to form peptide chains, thus preventing bacterial protein synthesis and limiting bacterial growth. However, tigecycline’s ability to bind with ribosomes is 5 times that of other tetracycline antibiotics, which means that tetracycline’s anti-drug resistance ability is stronger. Tigecycline’s structure is similar to that of minocycline, but tigecycline’s antibacterial activity is much stronger, and bacteria are less likely to develop resistance to it compared to other tetracycline drugs, and it can also act on the methicillin-resistant Staphylococcus aureus. Tigecycline’s antifungal spectrum includes gram-positive bacteria, gram-negative bacteria and anaerobic bacteria. In vitro experiments and clinical trials showed that tigecycline is sensitive to some aerobic gram-negative bacteria (such as Citrobacter freundii, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae and Klebsiella pneumoniae, Acinetobacter baumannii, Aeromonas hydrophila, Citrobacter Enterobacteriaceae, hemorrhagic Pasteurella, Serratia marcescens, and Stenotrophomonas maltophilia). Pseudomonas auruginosa is resistant to tigecycline.
| | Adverse reaction | The most common adverse effects are nausea and vomiting, which usually happens in the first 1-2 days of treatment and are mild to moderate in intensity. In a positive drug control clinical trial, 35% percent of complex skin and skin structure infection patients using tigecycline experienced nausea, and 20% experienced vomiting; vancomycin/aztreonam use caused 8.9% nausea and 4.2% vomiting. 25.3% of complex abdominal infection patients using tigecycline experienced nausea, and 19.5% experienced vomiting; vancomycin/aztreonam caused 20.5% nausea and 15.3% vomiting.
| | Description | The emergence of drug-resistant bacteria has diminished the clinical utility of the
tetracyclines. Research to circumvent the efflux and ribosomal protection mechanisms
of bacteria has led to the development of the glycylcyclines. Tigecycline is
the first glycylcycline antibiotic to launch for the parenteral treatment of
baterial infection, including complicated intra-abdominal and skin infections. Its mechanism of action involves inhibiting protein translation in bacteria by binding
to the 30S ribosomal subunit and blocking entry of amino-acyl tRNA molecules
into the A site of the ribosome to effectively prevent incorporation of amino acid
residues into elongating peptide chains. Presumably, ribosomal protection proteins
are ineffective against tigecycline due to its higher affinity for ribosomal binding
compared to tetracyclines (approximately 16-fold). In addition, tigecycline may be
resistant to efflux mechanisms by either their inability to translocate it across the
cytoplasmic membrane due to steric complications or simply by their failure to
recognize the molecule.
| | Description | Tigecycline is a broad-spectrum glycylcycline antibiotic that binds to the bacterial 30S ribosome, blocking the entry of transfer RNA, which halts protein synthesis and inhibits bacterial growth. It is active against a panel of 1,924 European clinical bacterial isolates including S. aureus, S. epidermidis, S. pneumoniae, E. faecalis, E. faecium, E. coli, K. pneumoniae, P. aeruginosa, and P. mirabilis strains (MICs = <1-32 μg/ml). In vivo, tigecycline (6.25 mg/kg twice daily for 5 days) decreases levels of C. difficile cytotoxin activity and spore formation in cecum and colon in a mouse model of C. difficile infection. Formulations containing tigecycline have been used in the treatment of a variety of bacterial infections. | | Chemical Properties | Orange Solid | | Originator | Wyeth (US) | | Uses | antineoplastic | | Uses | Tigecycline is a semi-synthetic tetracycline prepared by the introduction of a tert-butylaminoacetamido group into a previously unexplored and un-substituted region of existing tetracyclines. Like other tetracyclines, tigecycline acts by reversibly binding to the 30S ribosomal subunit and inhibits protein translation by blocking entry of aminoacyl-tRNA into the ribosome A site. The enhanced activity can be attributed to stronger binding affinity, thus minimising the impact of existing mechanisms of resistance. Tigecycline is regarded as the first of a new class of glycylcyline antibiotics. Critical comparison to the tetracycline class appears to be lacking in the literature. | | Uses | A broad spectrum glycylcycline antibiotic | | Uses | A glycylcycline antibiotic, used to treat infection by drug resistant bacteria such as Staphylococcus aureus (Staph aureus) and Acinetobacter baumannii. | | Definition | ChEBI: Tetracycline in which the hydroxy group at position 5 and the methyl group at position 6 are replaced by hydrogen, and with a dimethylamino substituent and an (N-tert-butylglycyl)amino substituent at positions 7 and 9, respe
tively. A glycylcycline antibiotic, it has activity against a broad range of Gram-positive and Gram-negative bacteria, including tetracycline-resistant organisms. It is used for the intravenous treatment of complicated skin and skin structure infections ca
sed by susceptible organisms. | | Brand name | Tygacil | | Antimicrobial activity | It is as potent as, or more potent than,
earlier tetracyclines and activity is retained against strains
expressing acquired tetracycline resistance determinants. It
displays better activity than tetracycline, doxycycline or
minocycline against Streptococcus spp. and against Enterococcus
faecalis and E. faecium. Among Gram-negative organisms it
displays improved activity against Citrobacter freundii,
Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae,
Salmonella spp., Serratia marcescens and Shigella spp. The
spectrum includes rapidly growing mycobacteria. Ps. aeruginosa,
Pr. mirabilis, other Proteus spp. and some strains of
Corynebacterium jeikeium are resistant. Activity against strains
expressing acquired resistance to earlier tetracyclines is
attributed to failure of the MFS efflux pumps to recognize
tigecycline, and to a novel mechanism of ribosome binding
that permits tigecycline to overcome ribosomal protection
mechanisms.
Comparative susceptibility data for some atypical pathogens
are not available. However, in common with earlier
tetracyclines,
it is active against Chlamydophila and Mycoplasma
spp. and rapidly growing Mycobacteria spp. It is less active
than minocycline or tetracycline against U. urealyticum. | | General Description | Tigecycline (Tygacil) is a first-in-class (a glycylcycline) intravenousantibiotic that was designed to circumvent manyimportant bacterial resistance mechanisms. It is not affectedby resistance mechanisms such as ribosomal protection, effluxpumps, target site modifications, β-lactamases, or DNAgyrase mutations. Tigecycline binds to the 30S ribosomalsubunit and blocks peptide synthesis. The glycylcyclinesbind to the ribosome with five times the affinity of commontetracyclines. Tigecycline also possesses a novel mechanismof action, interfering with the mechanism of ribosomal protectionproteins. Tigecycline, unlike common tetracyclines,is not expelled from the bacterial cell by efflux pumpingprocesses.
Tigecycline is recommended for the treatment of complicatedskin and skin structure infections caused by E. coli,E. faecalis (vancomycin-susceptible isolates), S. aureus(methicillin-susceptible and methicillin-resistant isolates),S. pyogenes, and B. fragilis among others. Tigecycline is alsoindicated for complicated intra-abdominal infections causedby strains of Clostridium, Enterobacter, Klebsiella, andBacteroides. To reduce the development of resistance to tigecycline,it is recommended that this antibiotic be used onlyfor those infections caused by proven susceptible bacteria.Glycylcyclines are structurally similar to tetracyclines,and appear to have similar adverse effects. These mayinclude photosensitivity, pancreatitis, and pseudotumorcerebri. Nausea and vomiting have been reported. | | Pharmaceutical Applications | 9-T-butylglycylamido-minocycline. A compound of the glycylcycline
class available as a powder for intravenous infusion. | | Pharmacokinetics | Cmax 100 mg intravenous infusion (1 h): 0.85–1 mg/L
Plasma half-life: 37–67 h
Volume of distribution: 7–10 L/kg
Plasma protein binding: 68%
Distribution and excretion
It is widely distributed and is concentrated in the gallbladder,
colon and lung. The volume of distribution is dose related and
variable, but is generally greater than that of older tetracyclines.
CSF penetration is poor. Tigecycline is excreted in the
feces and urine predominantly as the unchanged molecule.
The elimination half-life is long (37–67 h). Tigecycline clearance
is decreased by 20% in patients with renal failure. No
dosage adjustments are apparently necessary for tigecycline
in patients with renal impairment. | | Clinical Use | Complicated skin and skin structure infections
Complicated intra-abdominal infections
Community-acquired bacterial pneumonia
Recommended principally for the treatment of infections with
multiresistant organisms. | | Side effects | Side effects typical of the group, including nausea, vomiting,
diarrhea and headache, occur. Occasional cases of pancreatitis,
hypoproteinemia, antibiotic-associated colitis and thrombocytopenia
have also been reported. | | Synthesis | It
does not require dosage adjustment in patients with impaired
renal function and is conveniently dosed every 12 hours.
Synthesis of tigecycline started with nitration
of 138 with potassium nitrate and concentrated sulfuric
acid to give 9-nitro derivative 139 in 93 % yield as disulfate
salt, which was hydrogenated over Pd/C in 2-methoxyethanol/
2N sulfuric acid at 40 psi to provide 9-aminominocycline
(140). Finally, 9-aminominocycline (140) is acylated directly
with N-tert-butylglycyl chloride in a 1:5 mixture of acetonitrile
and N, N-dimethylpropyleneurea (DMPU) with anhydrous
sodium carbonate to give tigecycline (XX). 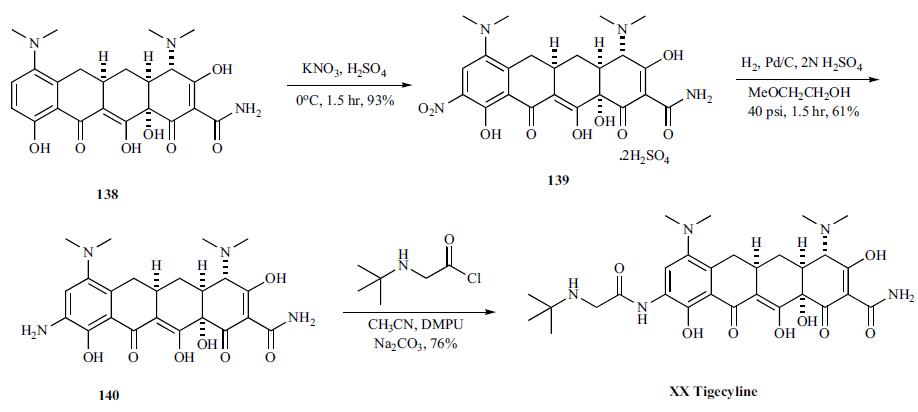
| | in vitro | tigecycline exihibited good in vitro activities. the range of mic90s was 0.12-0.5 μg/ml for vancomycin-susceptible and -resistant strains of enterococcus faecalis and enterococcus faecium [2]. tigecyclinewas concentrated in cells and eliminated primarily via biliary excretion. diminished renal function didn’t significantly alter its systemic clearance. tigecycline didn’t interfere with common cytochrome p450 enzymes, making pharmacokinetic drug interactions uncommon [3].the tissue penetration of tigecycline was excellent and the compound showed equivalence to imipenem/cilastatin in intra-abdominal infection and to vancomycin plus aztreonam in skin and skin structure infection [4]. | | in vivo | in an intraperitoneal systemic murine infection model, tigecycline exihibited in vivo activities against gisa, methicillin-susceptible s. aureus and methicillin-resistant s. aureus strains [2]. tigecycline and daptomycin showed similar in vivo efficacies against infections caused by the mssa strain (strain gc 4543) with the ed50s of 0.12 and 0.24 mg/kg, respectively. the ed50s of tigecycline was 0.72 mg/kg [2]. | | Drug interactions | Potentially hazardous interactions with other drugs
Anticoagulants: possibly enhanced anticoagulant
effect of coumarins.
Oestrogens: possibly reduced contraceptive effects of
oestrogens (risk probably small). | | Metabolism | Tigecycline is not thought to be extensively metabolised,
although some trace metabolites have been identified
including a glucuronide, an N-acetyl metabolite, and a
tigecycline epimer. Tigecycline is primarily eliminated
(about 60
%) via biliary excretion of unchanged drug and
some metabolites. | | References | 1) Greer (2006)?Tigecycline (Tygacil): the first in the glycylcycline class of antibiotics; Proc. (Bayl. Univ. Med. Cent.)?19?155
2) Peterson (2008)?A review of tigecycline – the first glycylcycline; Int. J. Antimicrob. Agents?32 Suppl 4?S215
3) Skrtic?et al.?(2011)?Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia; Cancer Cell?20?674
4) Jia?et al.?(2016)?Tigecyclin targets nonsmall cell lung cancer through inhibition of mitochondrial function; Fundam. Clin. Pharmacol.?30?297
5) Hu?et al.?(2016)?Antibiotic drug tigecycline inhibits melanoma progression and metastasis in a p21CIP1/Waf1-dependent manner; Oncotarget?7?3171
6) D’Andrea?et al.?(2016)?The mitochondrial translational machinery as a therapeutic target in Myc-driven lymphomas.; Oncotarget?7?72415
7) Chen?et al.?(2019)?Inhibition of mitochondrial translation selectively targets osteosarcoma; Biochem. Biophys. Res. Commun. 515 9 |
| | Tigecycline Preparation Products And Raw materials |
|



