|
| | (R*,S*)-(-)-8-Hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-2(1H)-quinolinone Basic information |
| Product Name: | (R*,S*)-(-)-8-Hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-2(1H)-quinolinone | | Synonyms: | (r*,s*)-(-)-8-hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-2(1h)-quinolinone;2(1h)-quinolinone,8-hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-,(r*,;8-hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-2(1h)-quinolinone(r*,s;s*)-(+-)-;ent-Florfenicol AMine-d3;(S)-N,N-DiMethyl-3-(1-naphthalenyl-d7-oxy)-3-(2-thienyl)propanaMine;ANTI-SARS VIRUS SM antibody produced in rabbit;Peplomer protein | | CAS: | 72332-33-3 | | MF: | C16H22N2O3 | | MW: | 290.36 | | EINECS: | 276-590-0 | | Product Categories: | | | Mol File: | 72332-33-3.mol |  |
| | (R*,S*)-(-)-8-Hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-2(1H)-quinolinone Chemical Properties |
| Melting point | 107-109°C | | storage temp. | -20°C | | solubility | Acetonitrile (Slightly), Chloroform (Slightly), Methanol | | form | Solid | | color | White to Off-White | | CAS DataBase Reference | 72332-33-3(CAS DataBase Reference) |
| | (R*,S*)-(-)-8-Hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-2(1H)-quinolinone Usage And Synthesis |
| Description | Like pirbuterol, procaterol exhibits similar broncholytic properties as albuteral, but it has
somewhat of a more prolonged action. It is recommended for use as an inhaled drug for
treating bronchial asthma. | | Chemical Properties | Off-White Solid | | Uses | A labelled intermediate of the enantiomer of Florfenicol (F405750), an antibacterial agent. | | Definition | ChEBI: 8-hydroxy-5-[1-hydroxy-2-(propan-2-ylamino)butyl]-1H-quinolin-2-one is a member of quinolines. | | Brand name | Pro-Air (Parke-Davis). | | Synthesis | Procaterol, 5-[1-hydroxy-2-[(1-methylethyl)amino]butyl]-8-hydroxy-2-(1H)
quinolone (23.3.25), is synthesized by acylation of 8-hydroxy-2(1H)-quinolone with 2-bromobutyric acid chloride at the fifth position of the quinoline system, which gives the
compound 23.3.23. This undergoes action of isopropylamine, forming an aminoketone
23.3.24, the carbonyl group of which is reduced by sodium borohydride, giving procaterol
(23.3.25) . 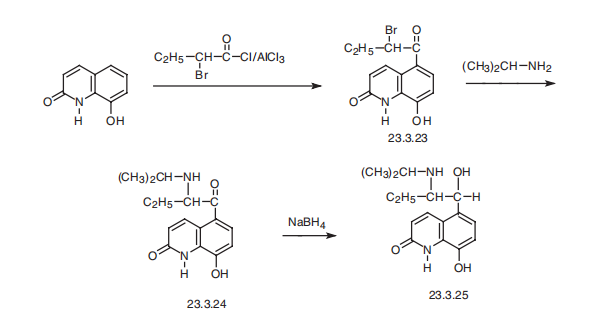
|
| | (R*,S*)-(-)-8-Hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-2(1H)-quinolinone Preparation Products And Raw materials |
|