|
| | IXABEPILONE Basic information |
| Product Name: | IXABEPILONE | | Synonyms: | IXABEPILONE;(1R,5S,6S,7R,10S,14S,16S)-6,10-dihydroxy-1,5,7,9,9-pentamethyl-14-[(E) -1-(2-methyl-1,3-thiazol-4-yl)prop-1-en-2-yl]-17-oxa-13-azabicyclo[14. 1.0]heptadecane-8,12-dione;Aza-epothilone B;Bms 247550-1;Ixempra;Ixempra kit;Unii-K27005np0a;Azi-epothilone B | | CAS: | 219989-84-1 | | MF: | C27H42N2O5S | | MW: | 506.7 | | EINECS: | 630-424-7 | | Product Categories: | API | | Mol File: | 219989-84-1.mol |  |
| | IXABEPILONE Chemical Properties |
| Melting point | >160°C (dec.) | | alpha | D22 -40.7° (c = 1.0 in chloroform) | | Boiling point | 697.8±55.0 °C(Predicted) | | density | 1.122±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | solubility | Chloroform (Slightly), Methanol (Slightly) | | pka | 13.73±0.70(Predicted) | | form | White solid. | | color | White to Off-White |
| | IXABEPILONE Usage And Synthesis |
| Description | Ixabepilone is an epothilone with broad-spectrum anticancer activity against a panel of 21 cancer cell lines (IC50s = 1.4-34.5 nM). It stabilizes and induces the polymerization of microtubules and induces cell cycle arrest during mitosis. Ixabepilone is cytotoxic to HCT116/VM46 and A2780Tax clonogenic cells, which are resistant to paclitaxel (; IC90s = 16 and 12.3 nM, respectively, for colony growth inhibition). In vivo, ixabepilone (6.3 and 10 mg/kg, i.v., respectively) shows antitumor activity in paclitaxel-resistant human Pat-21 breast carcinoma and HCT116/VM46 colon carcinoma mouse xenograft models with log cell kill (LCK) values of 1.6 and 2.4, respectively. Ixabepilone (10 mg/kg, i.v.) also increases the time for tumors to quadruple in volume by 7.2, 9, 6.5, 4.7, and >10 weeks, respectively, in mice implanted with Rh18 rhabdomyosarcoma, NB1643 neuroblastoma, WT5 Wilms'' tumor, OS29 osteosarcoma, and BT29 brain carcinoma cells. Formulations containing ixabepilone have used in the treatment of metastatic breast cancer. | | Description | Ixabepilone, a semisynthetic analog of epothilone B, was launched for the treatment of metastatic or locally advanced breast cancer. It is indicated
for use in combination with capecitabine in patients who have previously failed
treatment with an anthracycline such as doxorubicin and a taxane such as
paclitaxel. It is also approved as monotherapy for the treatment of metastatic or
locally advanced breast cancer in patients whose tumors are resistant or
refractory to anthracyclines, taxanes, and capecitabine. Ixabepilone is the first
member of the epothilone family of anticancer agents to be approved.
Epothilones are novel cytotoxic macrolides derived from bacterial fermentation.
Like the taxanes, their mechanism of action involves binding to and stabilizing microtubules, which results in mitotic arrest and apoptosis.
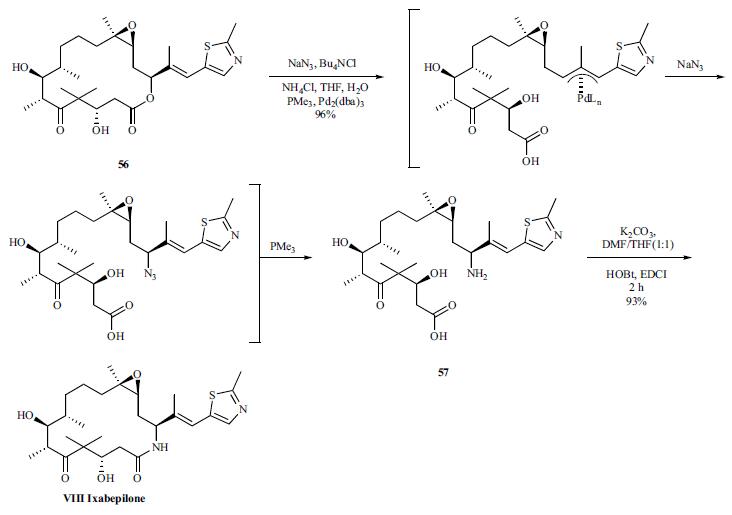
The most common adverse reactions (X20%) associated with ixabelipone as monotherapy or in combination with capecitabine were peripheral sensory neuropathy, fatigue/asthenia, myalgia/arthralgia, alopecia, nausea, vomiting, stomatitis/ mucositis, diarrhea, and musculoskeletal pain. The most common hematologic abnormalities (W 40%) include neutropenia, leukopenia, anemia, and thrombocytopenia. Ixabelipone in combination with capecitabine is contraindicated in patients with AST or ALT W2.5 ULN (upper limit of normal) or bilirubin W1 ULN due to increased risk of toxicity and neutropenia-related death. | | Originator | BMS (US) | | Uses | Ixabepilone is an anti-tumor agent used in the treatment of patients suffering from solid tumors, such as metastatic breast cancer. | | Uses | Antineoplastic; antimitotic. | | Definition | ChEBI: A macrocycle that is a lactam analogue of epothilone B. Binds directly to beta-tubulin subunits on microtubules, leading to suppression of microtubule dynamics. | | Brand name | Ixempra | | General Description | The epothilones are macrocyclic lactones that have a mechanismof action similar to that of the taxanes but offer severaladvantages.Ixabepilone is the semisyntheticamide analog of epothilone B that is isolated from themyxobacterium Sorangium cellulosum. The epothilonesshowed potent in vitro activity but greatly decreased activityin vivo caused by metabolic instability via hydrolysis ofthe macrocyclic lactone. Conversion to the lactam increasedstability and maintained in vivo activity. Ixabepilone hasbeen recently (2007) approved for the treatment of metastaticbreast cancer that is resistant to the taxanes. The agent isbelieved to bind to the same site occupied by the taxanes.Molecular modeling studies have been utilized to identifya common pharmacophore between the taxanes andepothilones.Like the taxanes, ixabepilone binds to β-tubulin and stabilizesmicrotubules resulting in cell death.The agent is usefulin cancers that have become resistant to the taxanes, becauseit is not removed by Pgp and is still capable of binding to altered beta tubulin to which the taxanes no longer bind.Increased water solubility also allows the agent to be administeredwithout the need for Cremophor EL, reducing thechance of hypersensitivity reactions. The current indicationsfor the agent are in metastatic breast cancer in combinationwith capecitabine after the failure of an anthracycline and ataxane and as monotherapy in metastatic breast cancer afterfailure of an anthracycline, a taxane, and capecitabine. Theagent is extensively metabolized in the liver primarily byCYP3A4 to give over 30 different metabolites. Eliminationoccurs primarily in the feces (65%) with a smaller amount(21%) occurring in the urine. The terminal elimination halflifeis 52 hours. Major toxicities associated with the useof ixabepilone have included peripheral neuropathy andmyelosuppression occurring as neutropenia. Occurring lessfrequently are alopecia, nausea, vomiting, mucositis, diarrhea,and muscle pain. | | Synthesis | The synthesis is described in the scheme, and was initiated by treating epothilone B (56) with sodium azide,
tetrabutylammonium chloride, ammonium chloride, trimethylphosphine
and tris-(dibenzylideneacetone)-dipalladium(0)
chloroform which gave the ring-opened amino acid 57 in
96% yield. It has been proposed that this reaction proceeds
via initial ring-opening -allyl palladium complex formation
followed by trapping with azide and subsequent reduction to
the desired amine. Lactamization of the acyclic amino
carboxylic acid 57 by reaction with K2CO3, HOBt and EDCI
provided ixabepilone (VIII) in 93% yield. Re-crystallization
from cyclohexane/ethyl acetate afforded ixabepilone in 56%
overall yield from epothilone B.
| | in vitro | in a large panel of tumor cell lines, bms-247550 was as active as epothilone b in inducing cytotoxicity. of the 21 cells lines tested, the ic50 values were in the range of 1.4–34.5 nm. bms-247550 almost completely blocked cells in mitosis as early as 8 h after the initiation of drug exposureat a concentration close to the ic90 (7.5 nm, clonogenic cytotoxicity assay) [2]. | | in vivo | bms-247550 has clearly demonstrated antitumor activity that is superior to paclitaxel in both paclitaxel-sensitive and -resistant tumors. bms-247550 was more efficacious than paclitaxel in all five paclitaxel-resistant tumors such as the clinically derived paclitaxel resistant pat-7 ovarian carcinoma, the a2780tax ovarian carcinoma that is resistant to paclitaxel because of tubulin mutations, the hct116/vm46 mdr colon carcinoma, the clinically derived paclitaxel-resistant pat-21 breast carcinoma, and the murine fibrosarcoma m5076. bms-247550 produced antitumor activity equivalent to paclitaxel against three paclitaxel-sensitive human tumor xenografts such as a2780 human ovarian carcinoma, hct116, and ls174t human colon carcinoma [2]. | | references | [1]. lee fy1, borzilleri r,fairchild cr, kim sh, long bh, reventos-suarez c, vite gd, rose wc, kramer ra.bms-247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. clin cancer res.2001 may;7(5):1429-37
[2]. denduluri n, low j a, lee j j, et al. phase ii trial of ixabepilone, an epothilone b analog, in patients with metastatic breast cancer previously untreated with taxanes[j]. journal of clinical oncology, 2007, 25(23): 3421-3427.
[3]. shannon puhalla, adam brufsky. ixabepilone: a new chemotherapeutic option for refractory metastatic breast cancer. biologics. 2008 sep; 2(3): 505–515. |
| | IXABEPILONE Preparation Products And Raw materials |
| Raw materials | 2-Oxiraneundecanoic acid, 3-[(2S,3E)-2-amino-3-methyl-4-(2-methyl-4-thiazolyl)-3-buten-1-yl]-β,ζ-dihydroxy-γ,γ,ε,η,2-pentamethyl-δ-oxo-, (βS,εR,ζS,ηS,2R,3S)--->Azacyclohexadec-13-ene-2,6-dione, 4,8-dihydroxy-5,5,7,9,13-pentamethyl-16-[(1E)-1-methyl-2-(2-methyl-4-thiazolyl)ethenyl]-, (4S,7R,8S,9S,13Z,16S)--->2-Oxiraneundecanoic acid, 3-[(2S,3E)-2-azido-3-methyl-4-(2-methyl-4-thiazolyl)-3-buten-1-yl]-β,ζ-dihydroxy-γ,γ,ε,η,2-pentamethyl-δ-oxo-, (βS,εR,ζS,ηS,2R,3S)--->277749-43-6-->224580-49-8-->277749-45-8-->Epothilone B-->277749-36-7 |
|



