|
| Product Name: | Alogliptin benzoate | | Synonyms: | (R)-2-((6-(3-aMinopiperidin-1-yl)-3-Methyl-2,4-dioxo-3,4-dihydropyriMidin-1(2H)-yl)Methyl)benzonitrile benzoate;Alogliptin benzoate 2-[[6-[(3R)-3-Amino-1-piperidinyl]-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl]methyl]benzonitrile benzoate;Alogliptin(Alogliptin benzoate;2-[6-[3(R)-AMinopiperidin-1-yl]-3-Methyl-2,4-dioxo-1,2,3,4-tetrahydropyriMidin-1-ylMethyl]benzonitrile benzoate Monobenzoate;Alogliptin API;ALOGLIPTIN(SYK-322) BENZOATE;(R)-2-((6-(3-Aminopiperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl)benz;2-[[6-[(3R)-3-aMino-1-piperidinyl]-3,4-dihydro-3-Methyl-2,4-dioxo-1(2H)-pyriMidinyl]Methyl]-Benzonitrile Monobenzoate | | CAS: | 850649-62-6 | | MF: | C25H27N5O4 | | MW: | 461.52 | | EINECS: | 691-730-4 | | Product Categories: | antidiabetic;Inhibitors;Final material;pharmaceutical intermediate;API;850649-62-6 | | Mol File: | 850649-62-6.mol |  |
| | Alogliptin benzoate Chemical Properties |
| Melting point | 180-182°C | | storage temp. | Refrigerator, under inert atmosphere | | solubility | Chloroform (Slightly, Heated), DMSO (Slightly, Heated), Methanol (Slightly, Heat | | form | Solid | | color | White to Off-White |
| | Alogliptin benzoate Usage And Synthesis |
| Indications and Usage | Alogliptin (benzoic acid) is a type-2 diabetes medication, and it is a type of serine protease dipeptidyl peptidase IV (DPP-4) inhibitor developed by the Japanese company Takeda. Alogliptin, used alone or in combination with other blood sugar-lowering medication, is usually well-tolerated in type-2 diabetes patients. This medication has a low risk of hypoglycemia, with Alogliptin treatment groups ≤8.3% and placebo groups ≤10.5%, and shows no difference between young and elderly patients. In addition to effectively lowering blood sugar, this medicine also lowers the risks of hypoglycemia and weight increase, overcoming great obstacles in patient treatment and providing new hope for diabetes treatment.
| | Mechanisms of Action | Alogliptin selectively inhibits DPP-4 to reduce the inactivation of glucagon-like peptide 1 (GLP-1) and increase the GLP-1 levels in the body, thus lowering blood sugar. Once blood sugar reaches normal levels, it will cease its sugar-lowering effects, thus effectively reducing the risks of hypoglycemia. Additionally, DPP-4 inhibitor also slows gastric emptying, increases the feeling of fullness, and controls appetite, thus helping patients control their weight.
| | Adverse reactions | Common side effects of Alogliptin include nasopharyngitis, headaches, and upper respiratory tract infection. Most side effects are light to moderate and are unrelated to dosage.
| | Uses | Alogliptin Benzoate is an oral antihyperglycemic agent that is a selective inhibitor of the enzyme dipeptidyl peptidase-4 (DPP-4). Alogliptin Benzoate is used in the treatment of type 2 diabetes. | | Definition | ChEBI: A benzoate salt obtained by combining equimolar amounts of alogliptin and benzoic acid. Used for treatment of type 2 diabetes. | | Biological Activity | alogliptin is a novel, highly selective and potent inhibitor of serine protease dipeptidylpeptidase-4 (dpp-4) with ic50 value of less than 10 nm [1].alogliptin has been reported to significantly reduce plasma dpp-4 activity and increase active glp-1 levels in a dose-dependent manner in ob/ob mice. besides, alogliptin after 4 weeks administration remarkably reduced non-fasting glycosylated hemoglobin, non-fasting plasma glucose and triglyceride levels, as well as siginificantly increased non-fasting plasma insulin and fasting pancreatic insulin content in ob/ob mice. moreover, alogliptin treated ob/ob mice have shown the increase of early-phase insulin secretion and the decrease of plasma glucose auc [2] | | Clinical Use | Alogliptin benzoate is a dipeptidyl peptidase IV (DPPIV) inhibitor discovered by Takeda
Pharmaceuticals and approved in Japan in 2010 for the treatment of type II diabetes mellitus.
Alogliptin is an oral drug for once a day dosing to complement diet and exercise. Alogliptin is the most
selective marketed DPPIV inhibition and has similar PK and PD properties compared to previous entries. The discovery, structure-activity relationship of related analogs, and synthesis of this
compound have been recently published. | | Synthesis | The most convenient synthesis for scale-up will be
highlighted from several published routes. Commercially available 2-cycanobenzyl
amine 1 was reacted with methylisocyanate in DCM at ambient temperature to provide N-methyl urea 2
in 85% yield. Reaction of the urea 2 with dimethyl malonate in refluxing ethanol with sodium ethoxide
as base gave the cyclized trione 3 in 78-85% yield. The trione 3 was then refluxed in neat POCl3 to
provide the penultimate chloride crude 4 in 95% yield which was reacted with Boc-protected diamine 5
in the presense of potassium carbonate in DMF to furnish alogliptin I in 93-96% yield. Treatment of
alogliptin with benzoic acid in ethanol at 60-70 ??C followed by crystallization delivered the desired
alogliptin benzoate (I). 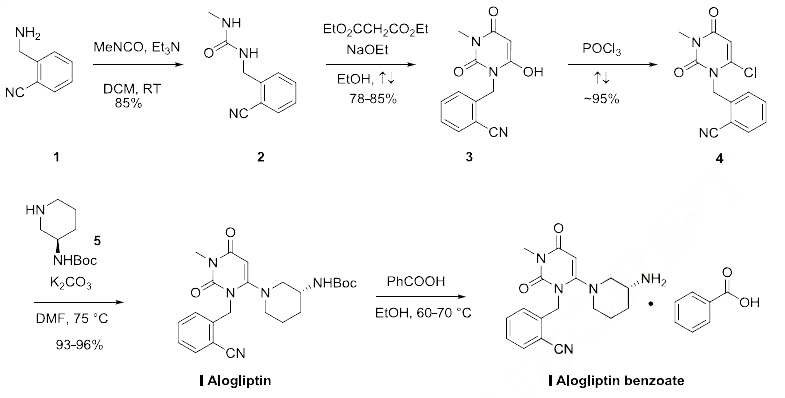
| | target | DPP-4 | | references | [1] feng j, zhang z, wallace mb, stafford ja, kaldor sw, kassel db, navre m, shi l, skene rj, asakawa t, takeuchi k, xu r, webb dr, gwaltney sl 2nd. discovery of alogliptin: a potent, selective, bioavailable, and efficacious inhibitor of dipeptidyl peptidase iv. j med chem. 2007 may 17;50(10):2297-300.
[2] moritoh y1, takeuchi k, asakawa t, kataoka o, odaka h. chronic administration of alogliptin, a novel, potent, and highly selective dipeptidyl peptidase-4 inhibitor, improves glycemic control and beta-cell function in obese diabetic ob/ob mice. eur j pharmacol. 2008 jul 7;588(2-3):325-32. |
| | Alogliptin benzoate Preparation Products And Raw materials |
|