|
| Product Name: | Clomifene | | Synonyms: | clomifene;Clomiphene;Clomiphene,E/Z-mixture;2-(4-[2-Chloro-1,2-diphenylethenyl]phenoxy)-N,N-diethylethanamine;CLOMIFENE (CLOMIPHENE);2-[4-(2-Chloro-1,2-diphenylvinyl)phenoxy]-N,N-diethylethan-1-amine;2-[4-(2-Chloro-1,2-diphenylvinyl)phenoxy]-N,N-diethylethanamine;Clomiphene B | | CAS: | 911-45-5 | | MF: | C26H28ClNO | | MW: | 405.96 | | EINECS: | 213-008-6 | | Product Categories: | | | Mol File: | 911-45-5.mol |  |
| | Clomifene Chemical Properties |
| Melting point | 117.25°C | | Boiling point | 509.0±50.0 °C(Predicted) | | density | 1.0166 (rough estimate) | | refractive index | 1.5790 (estimate) | | storage temp. | Amber Vial, -20°C Freezer | | solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) | | form | Solid | | pka | 9.54±0.25(Predicted) | | color | White to Pale Yellow | | NIST Chemistry Reference | Clomiphene(911-45-5) | | EPA Substance Registry System | Clomiphene (911-45-5) |
| | Clomifene Usage And Synthesis |
| Description | Clomiphene is an antiestrogen thought to increase sperm parameters in
males attempting to conceive. The objective of this review was to
evaluate the efficacy and safety of Clomiphene citrate in the treatment
of male patients with infertility. It is an oral agent used to treat
infertility in women desiring pregnancy. Clomiphene has been linked to a
low rate of transient serum aminotransferase elevations during therapy
and to rare instances of clinically apparent liver injury, which can be
severe and even fatal.A triphenyl ethylene stilbene derivative which is
an estrogen agonist or antagonist depending on the target tissue. | | Hormonal modulation | CLOMIPHENE exerts its effects centrally with a
result of increased LH and FSH secretion and increased testicular
testosterone production. Many studies have described significant
increases in serum testosterone, LH, and FSH with CLOMIPHENE treatment.
Studies have revealed comparable increases in serum testosterone levels
in hypogonadal men treated with CLOMIPHENE compared with TRT. CLOMIPHENE
has also been compared with aromatase inhibitors, such as anastrozole,
and CLOMIPHENE has proven to be more effective in increasing
testosterone levels. | | Originator | Clomid,Lepetit,Italy,1966 | | Uses | Clomifene is used for infertility in order to increase reproductive
properties of oligoovulatory women who have three or four ovulatory cycles per year,
leading to normal monthly ovulation. | | Uses | Antiestrogen. | | Indications | Clomifene acts by enhancing follicular growth caused by ovulation. The primary indication
for using clomifene is induction of ovulation in non-ovulating women who still have
some estrogen production. | | Definition | ChEBI: Zuclomifene is a stilbenoid. | | Manufacturing Process | A mixture of 20 g of 1-[p-(β-diethylaminoethoxy)phenyl]-1,2-diphenylethanol
in 200 cc of ethanol containing an excess of hydrogen chloride was refluxed 3
hours. The solvent and excess hydrogen chloride were removed under
vacuum, and the residue was dissolved in a mixture of ethyl acetate and
methylene chloride. 1-[p-(β-diethylaminoethoxy)phenyl]-1,2-diphenylethylene
hydrochloride was obtained, melting at 148° to 157°C. This hydrochloride salt
was treated with N-chlorosuccinimide in dry chloroform under reflux. The
product then obtained was converted to the free base and treated with citric
acid. The dihydrogen citrate salt of 1-[p-(β-diethylaminoethoxy)phenyl]-1,2-
diphenylchloroethylene was obtained, melting at 116.5° to 118°C.
The intermediate 1-[p-(β-diethylaminoethoxy)phenyl]-1,2-diphenylethanol was
obtained by treating 4-(β-diethylaminoethoxy)benzophenone with
benzylmagnesium chloride. It melted at 95° to 96°C. | | Brand name | Clomid (Sanofi Aventis); Serophene (Serono). | | Therapeutic Function | Antiestrogen | | Synthesis | Clomifene, 2-[p-(2-chloro-1,2-diphenylvinyl)phenoxy]triethylamine
(28.2.4), is synthesized from 4-hydroxybenzophenone by reacting it with 2-diethylaminoethylchloride
in the presence of an alkali, which gives 4-(2-diethylaminoethoxy)benzophenone
(28.2.1). This is reacted with benzylmagnesium chloride in a Grignard reaction,
forming as a result the corresponding carbinol (28.2.2). Dehydrating this with hydrogen chloride gives 2-[p-(1,2-diphenylvinyl) phenoxy]triethylamine (28.2.4), the vinylic hydrogen
atom of which is replaced with a chlorine atom using N-chlorosuccinimide, giving clomifene
(28.2.4) . 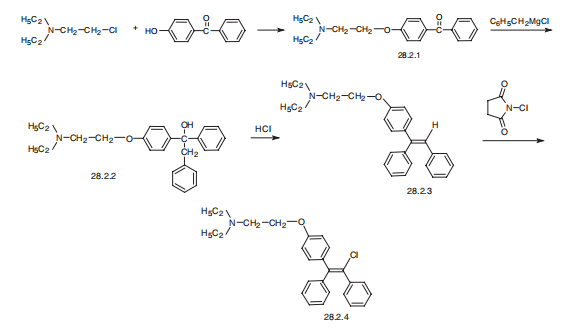
|
| | Clomifene Preparation Products And Raw materials |
|



