|
| | Tripelennamine Basic information |
| Product Name: | Tripelennamine | | Synonyms: | 1,2-Ethanediamine, N,N-dimethyl-N-(phenylmethyl)-N-2-pyridinyl-;PYRIBENZAMINE;Benzoxale;N,N-Dimethyl-N'-(phenylmethyl)-N'-(2-pyridinyl)-1,2-ethanediamine;Resistamine;Tonaril;Tripelenamine;N'-benzyl-N,N-dimethyl-N'-pyridin-2-ylethane-1,2-diamine | | CAS: | 91-81-6 | | MF: | C16H21N3 | | MW: | 255.36 | | EINECS: | 202-100-1 | | Product Categories: | | | Mol File: | 91-81-6.mol |  |
| | Tripelennamine Chemical Properties |
| Melting point | 25°C | | Boiling point | bp0.1 138-142°; bp1.7 185-190°; bp20 193-205° | | density | 1.0683 (rough estimate) | | refractive index | nD25 1.5759-1.5765 | | pka | pKa 3.90±0.08(H2O
t undefined
I = 0.30
(NaCl)) (Uncertain);8.68±0.06(H2O
t undefined
I = 0.30
(NaCl)) (Uncertain) | | Water Solubility | 587.3mg/L(30 ºC) | | Stability: | Stable. Incompatible with strong oxidizing agents. | | EPA Substance Registry System | Tripelennamine (91-81-6) |
| | Tripelennamine Usage And Synthesis |
| Chemical Properties | White, bitter, crystalline powder. Solutions
are acid to litmus.Soluble in water
and alcohol; very slightly soluble in ether; practically
insoluble in chloroform and benzene; 1%
solution in water has a pH of 4.3. | | Originator | Pyribenzamine,Ciba,US,1946 | | Uses | Medicine (antihistamine, sunburn treatment). | | Uses | This drug lessens the allergic response of the organism caused by histamine. Tripelennamine
is used for allergic symptoms, rhinitis, conjunctivitis, and for allergic and anaphylactic reactions.
Synonyms of this drug are pelanin and pyribenzamine. | | Definition | ChEBI: Tripelennamine is an aromatic amine. | | Manufacturing Process | 46 g of α-benzylaminopyridine in 50 cc of dry toluene are heated to 80°C [the
α-benzylaminopyridine may be obtained either according to the method of
Tchitchibabine and Knunjanz, Berichte, 64, 2839 (1931), which consists in
warming α-aminopyridine with benzaldehyde in formic acid, or alternatively by
the action of benzyl chloride on sodio-α-aminopyridine]. To the toluene
solution there are added gradually 9.5 g of 85% sodamide. After evolution of
ammonia, the major part of the toluene is distilled off; into the pasty mass
which remains there are poured 120 cc of an ethereal solution of 27 g of
dimethylaminochloroethane.
The mixture is heated until the temperature reaches 140°C, the ether distilling
out, then finally heated under reduced pressure (150 mm Hg) for 1/2 hour.
The mass is taken up with dilute hydrochloric acid and ether, neutralized at pH
7, and α-benzylaminopyridine separates. After making alkaline, using excess of potash, it is extracted with benzene, dried and distilled. The product
thereby obtained, dimethylamino-ethyl-N-benzyl-N-α-arninopyridine, boils at
135° to 190°C/1.7 mm, according to US Patent 2,502,151. | | Brand name | PBZ (Novartis). | | Therapeutic Function | Antihistaminic | | Reactivity Profile | Tripelennamine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. | | Health Hazard | SYMPTOMS: Symptoms of exposure to Tripelennamine may include euphoria, aplastic anemia, excitement, hallucinations, ataxia, incoordination, athetosis, convulsions, postictal depression, dry mouth, fixed dilated pupils, flushing of the face, fever, central nervous system depression, drowsiness and coma. | | Fire Hazard | Flash point data for Tripelennamine are not available. Tripelennamine is probably combustible. | | Safety Profile | Poison by ingestion and
intraperitoneal routes. Human mutation data
reported. Has been implicated in aplastic
anemia. Used as an antdustasnine. Addicts
have added it to paregoric to make "blue
velvet," whtch can cause euphoria by
injection. When heated to decomposition it
emits toxic fumes of NOx. | | Synthesis | Tripelennamine, N-benzyl-N??,N??-dimethyl-N-2-pyridylethylenediamine
(16.1.6), is synthesized by reacting 2-benzylaminopyrridine (16.1.5) with 2-dimethylaminoethylchloride
in the presence of sodium amide. 2-Benzylaminopyrridine, in turn,
can be easily synthesized by reduction of a Schiff base, synthesized by condensation of
2-aminopyrridine with benzaldehyde. 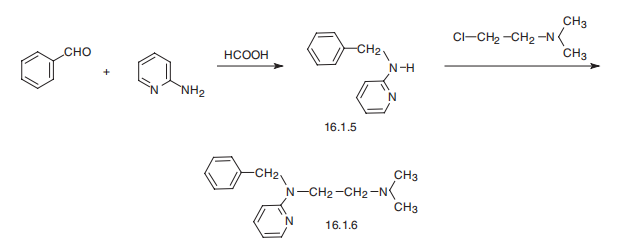
| | Metabolic pathway | When pyribenzamine is
incubated with rat liver microsomes, it is metabolized via N-oxide formation and
N-dealkylation which includes removal of the
dimethylamino moiety, the thiophenylmethyl moiety of
methaphenilene, and the benzyl moiety of
pyribenzamine. |
| | Tripelennamine Preparation Products And Raw materials |
|



