|
| | cis-1,2,3,6-Tetrahydrophthalic anhydride Basic information |
| Product Name: | cis-1,2,3,6-Tetrahydrophthalic anhydride | | Synonyms: | 1,3-isobenzofurandion,3a,4,7,7a-tetrahydro-,cis-;3-Isobenzofurandione,3a,4,7,7a-tetrahydro-,cis-1;4,7,7a-tetrahydro-3-isobenzofurandioncis-3a;cis-3a,4,7,7a-Tetrahydro-1,3-isobenzofurandione;CIS-THPA;CIS-DELTA4-TETRAHYDROPHTHALIC ANHYDRIDE;CIS-4-TETRAHYDROPHTHALIC ANHYDRIDE;CIS-1,2,3,6-TETRAHYDROPHTHALIC ANHYDRIDE | | CAS: | 935-79-5 | | MF: | C8H8O3 | | MW: | 152.15 | | EINECS: | 213-308-7 | | Product Categories: | Diels-Alder Adducts | | Mol File: | 935-79-5.mol |  |
| | cis-1,2,3,6-Tetrahydrophthalic anhydride Chemical Properties |
| Melting point | 98 °C | | Boiling point | 234.6°C (rough estimate) | | density | 1.2143 (rough estimate) | | vapor density | 5.2 (vs air) | | vapor pressure | <0.01 mm Hg ( 20 °C) | | refractive index | 1.4447 (estimate) | | Fp | 156 °C | | storage temp. | under inert gas (nitrogen or Argon) at 2-8°C | | solubility | soluble in Toluene,Benzene | | form | powder to crystal | | color | White to Almost white | | Water Solubility | REACTS | | Sensitive | Moisture Sensitive | | BRN | 82341 | | Exposure limits | ACGIH: TWA 0.01 mg/m3
OSHA: TWA 0.25 ppm(1 mg/m3)
NIOSH: IDLH 10 mg/m3; TWA 0.25 ppm(1 mg/m3) | | CAS DataBase Reference | 935-79-5(CAS DataBase Reference) | | NIST Chemistry Reference | cis-1,2,3,6-Tetrahydrophthalic anhydride(935-79-5) | | EPA Substance Registry System | 1,3-Isobenzofurandione, 3a,4,7,7a-tetrahydro-, (3aR,7aS)-rel- (935-79-5) |
| | cis-1,2,3,6-Tetrahydrophthalic anhydride Usage And Synthesis |
| Description | Tetrahydrophthalic anhydride is an organic compound with the formula C6H8C2O3. The compound exists as two isomers, this article being focused on the more common cis isomer. It is a precursor to other compounds including the dicarboxylic acid tetrahydrophthalic acid as well the tetrahydrophthalimide, which is a precursor to the fungicide Captan. It is a white solid that is soluble in organic solvents.
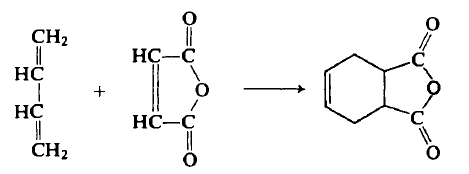
Tetrahydrophthalic anhydride, the cis isomer, is prepared by the Diels-Alder reaction of butadiene and maleic anhydride. | | Chemical Properties | Tetrahydrophthalic Anhydride (THPA) is a white crystalline powder or FLAKES. It is slightly soluble in benzene and acetone and it is also sensitive to moisture. | | Uses | cis-1,2,3,6-Tetrahydrophthalic anhydride (THPA) can be used as:
- A curing agent for epoxides.
- A chemical modifier in the modification of polystyrene via Friedel-Crafts acylation for enhancing the thermal stability of the polymer.
- A reactant for the synthesis of cis-tetrahydroisoindole-1,3-dione derivatives and cyclic diimides.
| | Uses | Tetrahydrophthalic Anhydride is an anhydride hardener mainly used in the fields of coatings, epoxy resin curing agents, polyester resins, adhesives, plasticizers, pesticides, etc. It is also used as a chemical intermediate for light colored alkyds.
Tetrahydrophthalic Anhydride is an organic compound obtained from the reaction of Maleic Anhydride and Butadiene. | | Preparation | A flask containing 500 ml of dry benzene and 196 gm (2 moles) of maleic anhydride is heated with a pan of hot water while butadiene is introduced rapidly (0.6-0.8 liter/min) from a commercial cylinder. The solution is stirred rapidly and the heating is stopped after 3-5 min when the temperature reaches 50°C. In 15-25 min the reaction causes the temperature of the solution to reach 70-75°C. The absorption of the rapid stream of butadiene is nearly complete in 30-40 min. The addition of butadiene is continued at a slower rate for a total of 2\ hr. The solution is poured into a 1-liter beaker which is covered and kept at 0-5°C overnight. The product is collected on a large Buchner funnel and washed with 250 ml of b.p. 35-60°C petroleum ether. A second crop is obtained by diluting the filtrate with an additional 250 ml of petroleum ether. Both crops are dried to constant weight in an oven at 70-80°C to yield 281.5-294.5 gm (96- 97%, m.p. 99-102°C). Recrystallization from ligroin or ether raises the melting point to 103-104°C.
| | Synthesis Reference(s) | Journal of the American Chemical Society, 64, p. 802, 1942 DOI: 10.1021/ja01256a018 |
| | cis-1,2,3,6-Tetrahydrophthalic anhydride Preparation Products And Raw materials |
| Raw materials | Maleic anhydride-->1,3-Butadiene-->4-bromo-1,2-butadiene-->3-SULFOLENE | | Preparation Products | N-Hydroxymethyl-3,4,5,6-tetrahydrophthalimide-->Captan-->1,2,3,4-Butanetetracarboxylic acid-->Di-(2-Ethylhexyl)4,5-Epoxytetrahydrophthalate-->4-Methylphthalic acid-->CIS-4-CYCLOHEXENE-1,2-DICARBOXYLIC ACID-->CIS-1,2-CYCLOHEXANEDICARBOXYLIC ANHYDRIDE-->1H-Isoindole-1,3(2H)-dione, 3a,4,7,7a-tetrahydro-2-(2-hydroxyethyl)-, (3aR,7aS)-rel--->dimethyl cyclohex-3-ene-1,6-dicarboxylate |
|



