|
| | Diltiazem Basic information |
| Product Name: | Diltiazem | | Synonyms: | (+)-5-[2-(Dimethylamino)ethyl]-cis-2,3-dihydro-3-hydroxy-2-(p-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one acetate (ester);(2s-cis)-dro-2-(4-methoxyphenyl);(2S,3S)-2,3-Dihydro-2-(4-methoxyphenyl)-3-hydroxy-5-[2-(dimethylamino)ethyl]-1,5-benzothiazepine-4(5H)-one acetate;(2R)-3α-(Acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2α-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one;[2R,(+)]-3α-(Acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2α-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one;3α-(Acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2α-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one;3α-Acetyloxy-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2α-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one;[(2S,3S)-5-(2-dimethylaminoethyl)-2-(4-methoxyphenyl)-4-oxo-2,3-dihydro-1,5-benzothiazepin-3-yl] acetate | | CAS: | 42399-41-7 | | MF: | C22H26N2O4S | | MW: | 414.52 | | EINECS: | 255-796-4 | | Product Categories: | 42399-41-7 | | Mol File: | 42399-41-7.mol |  |
| | Diltiazem Chemical Properties |
| | Diltiazem Usage And Synthesis |
| Originator | Herbesser,TANABE SEIYAKU,Japan,1974 | | Uses | It is used for stable and nonstable angina pectoris (including after myocardial infarctions)
as well as in arterial hypertension. | | Uses | Diltiazem is a potent vasodilator compound. Antihypertensive. | | Definition | ChEBI: A 5-[2-(dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydro-1,5-benzothiazepin-3-yl acetate in which both stereocentres have S configuration. A calcium-channel blocker and vasodilator, it is used as the hydrochloride in the m
nagement of angina pectoris and hypertension. | | Manufacturing Process | β-Diethylaminoethyl chloride is condensed with 2-(4-methoxyphenyl)-3-
hydroxy-2,3-dihydro-1,5-benzothiazepin-4(5H)-one in a first step. Then a
mixture of 1.5 grams of 2-(4-methoxyphenyl)-3-hydroxy-5-(βdimethylaminoethyl)-2,3-dihydro-1,5-benzothiazepin-4(5H)-one and 20 ml of
acetic anhydride was heated on a water bath for 5 hours. The reaction
mixture was evaporated under reduced pressure to remove acetic anhydride
and the concentrated product was poured into ice water. The resulting mixture
was made alkaline with sodium bicarbonate and extracted with chloroform.
The chloroform layer was dried and evaporated to remove the solvent. The
residue was dissolved in acetone, and an ethanol solution containing hydrogen
chloride was added thereto producing 1.53 grams of 2-(4-methoxyphenyl)3-
acetoxy-5-(β-dimethylaminoethyl)-2,3-dihydro-1,5-benzothiazepin-4(5H)-one
hydrochloride having a melting point from 187° to 188°C.
The starting material is made by reacting 4-methoxybenzaldehyde with ethyl
chloroacetate; that product with sodium ethoxide; and that product with 2-
aminothiophenol. | | Therapeutic Function | Coronary vasodilator | | Mechanism of action | Diltiazem reduces the transmembrane influx of calcium ions into cells of cardiac muscle and smooth musculature of vessels. It causes dilation of coronary and peripheral vessels, increases coronary blood flow, and prevents development of coronary artery spasms. It lowers elevated arterial pressure and reduces tachycardia. | | Clinical Use | The antiarrhythmic actions and uses of diltiazem are similar to those of verapamil.
Diltiazem is effective in controlling the ventricular
rate in patients with atrial flutter or atrial fibrillation. | | Synthesis | Diltiazem, 5-[2-(diethylamino)ethyl]-cis-2,3-dihydro-3-hydroxy-2-
(4-methoxy-phenyl)-1,5-benzothiazepin-4(5H)-one (19.3.10), is synthesized in the following
manner. The condensation of 4-methoxybenzaldehyde with methylchoroacetate in the presence
of sodium methoxide in Darzens reaction conditions gives methyl ester of 3-
(4-methoxyphenyl)-glycidylic acid (19.3.5). Reacting it with 2-aminothiophenol with the
opening of epoxide ring gives methyl ester of 2-hydroxy-3-(2'-aminophenylthio)-3- (4"-
methoxyphenyl)propionic acid (19.3.6). Hydrolysis of the resulting compound with alkali
leads to the formation of the corresponding acid (19.3.7) in the form of a racemic mixture,
which when on interaction with (+)-|á-phenylethylamine gives threo-(+)-2-hydroxy-3-(2'-
aminophenylthio)-3-(4"-methoxyphenylpropionic acid (19.3.8). Boiling this in a mixture of
acetic anhydride/dimethylformamide/pyridine system brings to cyclization to the thiazepine
ring and simultaneously acylates the hydroxyl group, forming (+)-cis-2-(4-methoxyphenyl)-
3-acetoxy-2,3-dihydro-1,5-benzothiazepin-4-(5H)-one (19.3.9). Alkylation of the resulting
product with 2,2-dimethylaminoethylchloride forms diltiazem (19.3.10). 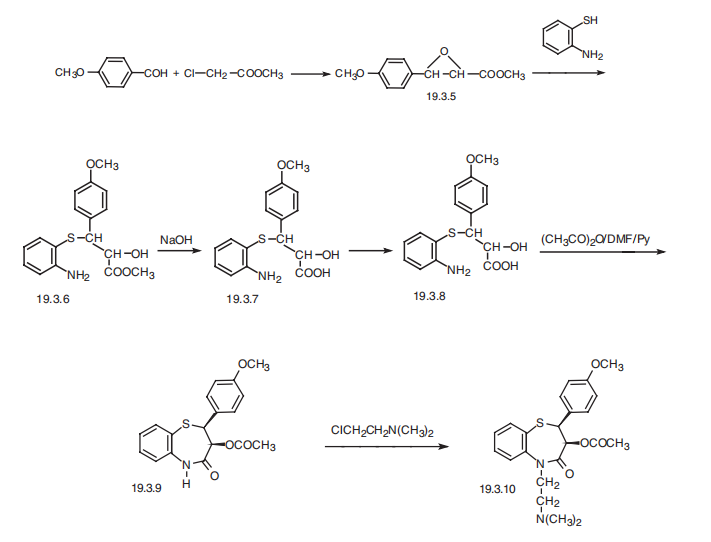
|
| | Diltiazem Preparation Products And Raw materials |
|



