|
| | Phenylboronic acid Basic information |
| | Phenylboronic acid Chemical Properties |
| Melting point | 216-219 °C(lit.) | | Boiling point | 265.9±23.0 °C(Predicted) | | density | 1.13±0.1 g/cm3(Predicted) | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) | | pka | 8.83(at 25℃) | | form | Crystalline Powder | | color | White to off-white | | Water Solubility | 10 g/L (20 ºC) | | Sensitive | Hygroscopic | | Merck | 14,1068 | | BRN | 970972 | | Stability: | Hygroscopic | | InChIKey | HXITXNWTGFUOAU-UHFFFAOYSA-N | | CAS DataBase Reference | 98-80-6(CAS DataBase Reference) | | EPA Substance Registry System | Boronic acid, phenyl- (98-80-6) |
| Hazard Codes | Xn,Xi,N | | Risk Statements | 22-36/37/38-20/21/22 | | Safety Statements | 22-24/25-36/37/39-26-36 | | WGK Germany | 3 | | RTECS | CY8575000 | | Hazard Note | Harmful | | TSCA | Yes | | HazardClass | IRRITANT, MOISTURE SENSITIVE | | HS Code | 29310095 | | Toxicity | LD50 orl-rat: 740 mg/kg 14KTAK -,693,64 |
| | Phenylboronic acid Usage And Synthesis |
| Chemical Properties | white to light yellow crystal powder. Insoluble in water and benzene, easily soluble in ether and methanol. When exposed to air or under heating conditions, it is easy to dehydrate and form a three-molecular polymer. | | Uses | Reagent used for• ;Rhodium-catalyzed intramolecular amination1 • ;Pd-catalyzed direct arylation2 • ;Mizoroki-Heck and Suzuki-Miyaura coupling reactions catalyzed by palladium nanoparticles3 • ;Palladium-catalyzed stereoselective Heck-type reaction4 • ;Highly effective Palladium-catalyzed arylation Suzuki-Miyaura cross-coupling in water5 Reagent used in Preparation of• ;Ni(II) pincer complex and Pd(II) pyridoxal hydrazone metallacycles as catalysts for the Suzuki-Miyaura cross-coupling reactions6,7• ;N-type polymers for all-polymer solar cells8 • | | Uses | Phenylboronic Acid is a compound used in organic synthesis of various pharmaceutical goods.
Phenylboronic acid (PBA) and its derivatives can bind sugars and other diol compounds to form cyclic boronate esters. These esters bear negative charges. Due to this reaction, sugars can be detected by means of electrochemical and optical techniques.
PBA modified electrodes have been constructed as reagentless electrochemical sensors. There, PBA is absorbed on the electrode surface either as a self- assembled monolayer film ore a polymer thin film. Basically, for optical detection techniques for the detection of sugars, PBA is modified with a chromophore that should change its properties on reaction with a sugar.
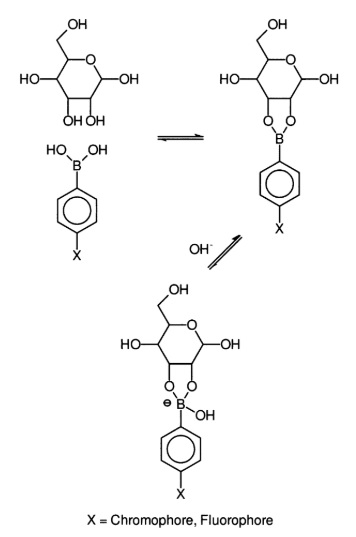
Reaction of Phenylboronic acid with a sugar
| | Uses | Phenylboronic acid and its derivatives form complexes with polyol compounds such as saccharides and poly(vinyl alcohol) (PVA). Glucose-sensitive hydrogels can be prepared by using phenylhoronic acid as a ligand for target glucose. For example. a copolymer with phenylboronic acid moieties was synthesized by copolymerization of 4V-vinyl-2-pyrrolidone (NVP) and 3-(acryl- amide)phcnylhoronic acid (PBA). The complexes between the NVP-PBA copolymer and PVA enable us to construct glucose-sensitive insulin release systems because the complexes dissociated in response to the glucose concentration.
This phenylboronic acid is soluble in water and the coupling reaction can even be carried out in water. It is compatible with many organic substituents such as esters, ketones, alcohols, etc. which would prohibit the use of Grignard reagents in coupling reactions.
| | Definition | ChEBI: Phenylboronic acid is a member of boronic acids. It is functionally related to a boronic acid. | | Synthesis Reference(s) | Organic Syntheses, Coll. Vol. 4, p. 68, 1963
The Journal of Organic Chemistry, 49, p. 5237, 1984 DOI: 10.1021/jo00200a045 | | General Description | Phenylboronic acid (PBA) is an organoboronic acid. It behaves as a molecular receptor that can attach to compounds containing cis-diol group. Microwave-assisted Suzuki coupling of aryl chlorides with phenylboronic acid in the presence of Pd/C (catalyst) and water (solvent) has been described. Palladium-catalyzed cross-coupling reaction of phenylboronicacid with haloarenes to afford biaryls has been reported. | | Safety Profile | Poison by intravenous andintraperitoneal routes. Moderately toxic by ingestion.Mildly toxic by skin contact. When heated to decomposition it emitsacrid smoke and irritating fumes. |
| | Phenylboronic acid Preparation Products And Raw materials |
|



