|
| | (E)-2-oxopropanal oxime Basic information |
| Product Name: | (E)-2-oxopropanal oxime | | Synonyms: | ANTI-PYRUVIC ALDEHYDE 1-OXIME;ISONITROSOACETONE;2-oxopropionaldehyde 1-oxime;PYRUVALDEHYDE 1-OXIME 98+%;2-OXO-PROPIONALDEHYDE OXIME;Propanal, 2-oxo-, 1-oxime;1-(Hydroxyimino)acetone;1-Hydroxyimino-2-propanone | | CAS: | 306-44-5 | | MF: | C3H5NO2 | | MW: | 87.08 | | EINECS: | 206-184-0 | | Product Categories: | | | Mol File: | 306-44-5.mol |  |
| | (E)-2-oxopropanal oxime Chemical Properties |
| Melting point | 69° | | Boiling point | 160.87°C (rough estimate) | | density | 1.0744 | | refractive index | 1.4940 (estimate) | | solubility | Chloroform (Slightly), Methanol (Slightly) | | pka | pK (25°) 8.39 | | form | Solid | | color | Brown | | EPA Substance Registry System | Propanal, 2-oxo-, 1-oxime (306-44-5) |
| | (E)-2-oxopropanal oxime Usage And Synthesis |
| Uses | (E)-2-oxopropanal oxime can be used as organic synthesis intermediates and pharmaceutical intermediates, mainly used in laboratory research and development processes and pharmaceutical and chemical production processes. | | Preparation | To a well-stirred, ice-cooled solution of 5.8 gm (0.1 mole) of acetone in 30 ml of glacial acetic acid is added dropwise a concentrated aqueous solution containing 15 gm of sodium nitrite. Stirring at 0°C is continued for 45 min, then 100 ml of water is added and the crude product is extracted with ether.
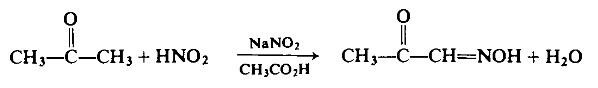
The ether extracts are combined, washed repeatedly with 10 ml portions of water, and dried with sodium sulfate. The ether is evaporated off under reduced pressure and the residue is dried on a porous plate. After recrys-tallization from benzene, 6 gm (69%) of the product is isolated, m.p. 65°C.
| | Synthesis | 
under the condition of the ice, to 1000 ml three port successively added in the bottle KOH (58.35g, 1 . 16eq, 1 . 04mol) and 585 ml water, the reaction system is cooled to 0 °C, adding to the reaction system (103.61g, 1eq, 0.892mol), after stirring at room temperature add 24h; once again the reaction system is cooled to 0 °C, in the reaction system by adding NaNO2(71.76g, 1 . 16eq, 1 . 04mol), the reaction system to maintain 0 °C, to wherein the dropwise H2SO4(50%), the for PH 4-5 (adding sulphuric acid to the volume to the final system is PH), stir at room temperature 2h, this will produce a large amount of gas in the process, a white precipitate, then in to the system by adding NaOH (35%) solution, the for PH 9-10, finally in the reaction system by adding 100 ml of toluene, the organic phase and aqueous phase separation, is added to the aqueous phase H2SO4(50%) the adjusted to PH 5-6, ethyl acetate (3*100 ml) extraction, anhydrous sodium sulfate for drying, can be obtained turns on lathe does white solid 61 . 3g (yield 79%). |
| | (E)-2-oxopropanal oxime Preparation Products And Raw materials |
|