|
| Product Name: | Oritavancin Diphosphate | | Synonyms: | Oritavancin Diphosphate;LY 333328 diphosphate;Oritavancin (phosphate);oritavancin,LY333328 diphosphate salt;LY 333328;ORITAVANCIN (PHOSPHATE);OC/ QTH05;Oritavancin diphosphate (LY333328);Vancomycin, 22-O-(3-amino-2,3,6-trideoxy-3-C-methyl-α-L-arabino-hexopyranosyl)-N3''-[(4'-chloro[1,1'-biphenyl]-4-yl)methyl]-, (4''R)-, phosphate (1:2) | | CAS: | 192564-14-0 | | MF: | C86H103Cl3N10O34P2 | | MW: | 1989.091142 | | EINECS: | | | Product Categories: | Inhibitors | | Mol File: | 192564-14-0.mol |  |
| | Oritavancin Diphosphate Chemical Properties |
| Melting point | >229°C (dec.) | | storage temp. | -20°C | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | powder | | color | white to beige |
| | Oritavancin Diphosphate Usage And Synthesis |
| Clinical Effects | Oritavancin diphosphate will most probably be prescribed as a once-daily dose and it demonstrates concentration-dependent bactericidal activity. Various clinical studies of oritavancin diphosphate have shown good performance. Oritavancin diphosphate has demonstrated preliminary safety and efficacy in Phase I and II clinical trials. In a Phase III clinical trial, oritavancin diphosphate has achieved the primary efficacy end point in the treatment of complicated Gram-positive skin and skin-structure infections. To date, adverse events have been mild and limited; the most common being administration site complaints, headache, rhinitis, dry skin, pain, increases in liver transaminases and accumulation of free cholesterol and phospholipids in phagocytic (macrophages) and nonphagocytic (fibroblast) cells.
| | Description | Oritavancin diphosphate is a glycopeptide analog related to the
vancomycin class of antibiotics that inhibits the transpeptidase
and transglycosylation steps of bacterial peptidoglycan cell-wall
synthesis. The compound was discovered and initially developed
by Eli Lilly and Co. The development of the compound was
sold to Intermune and then to Targanta which was acquired by
The Medicines Company who achieved approval of the drug by
the US FDA for the treatment of acute bacterial skin and skin-structure
infections caused by gram-positive bacteria. It is also
approved for the treatment of methicillin-resistant Staphylococcus
aureus (MRSA) infections. | | Uses | Antibacterial (peptidoglycan synthesis inhibitor). | | Uses | Oritavancin phosphate can be used in biological study of synthesis, properties, and mechanism of action of new generation of polycyclic glycopeptide antibiotics. | | Definition | ChEBI: A phosphate salt obtained by combining oritavancin with two molar equivalents of phosphoric acid. Used for the treatment of acute bacterial skin and skin structure infections caused or suspected to be caused by susceptible isolates of designated Gram-posit
ve microorganisms. | | Biochem/physiol Actions | Oritavancin is a lipoglycopeptide vancomycin analog with broad spectrum activity against gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA) and organisms resistant to vancomycin and other antibiotics such as linezolid and daptomycin. Oritavancin has multiple mechanisms of action, including inhibition of transglycosylation, inhibition of transpeptidation, and cell membrane interaction/disruption. Oritavancin also has a long half-life, allowing for a single intravenous dose of rather than the standard vacomycin treatment of twice-daily infusions for ten days. | | Synthesis | Commercial eremomycin (217) was treated
with 40-chlorobiphenylcarboxaldehyde (218) followed by sodium
cyanoborohydride in refluxing methanol to give oritavancin. Interestingly,
there are three amino groups within eremomycin that can
undergo reductive alkylation, and this chemistry preferentially
occurs at the disaccharide amino group. The reaction is reported
to occur in 69% crude yield and giving 16¨C18% yield of oritavancin
after high performance liquid chromatography (HPLC) purification.
No experimental details were found describing the preparation of
the diphosphate salt, but presumably this occurs through treatment
with phosphoric acid and crystallization to give oritavancin
diphosphate (XXVI). 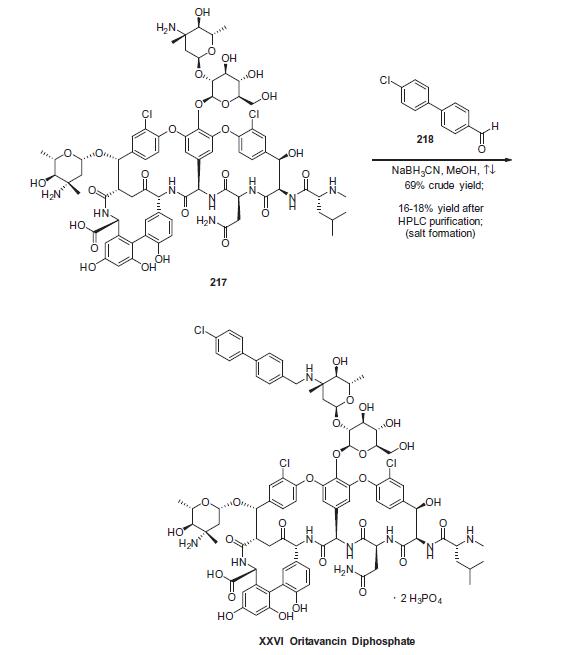
|
| | Oritavancin Diphosphate Preparation Products And Raw materials |
|