|
| | DEGARELIX Basic information |
| Product Name: | DEGARELIX | | Synonyms: | DEGARELIX;Degarelix acetate;N-Acetyl-3-(2-naphthyl)-D-alanyl-4-chloro-D-phenylalanyl-3-(3-pyridyl)-D-alanyl-L-seryl-4-[2,6-dioxohexahydropyrimidin-4(S)-ylcarboxamido]-L-phenylalanyl-4-ureido-D-phenylalanyl-L-leucyl-N6-isopropyl-L-lysyl-L-prolyl-D-alaninamide acetate;Degarelix acetate salt;D-Alaninamide, N-acetyl-3-(naphtalen-2-yl)-D-alanyl-4-chloro-D-phenylalanyl-3-(pyridin-3-yl)-D-alanyl-L-seryl-4-((((4S)-2,6-dioxohexahydropyrimidin-4-yl)carbonyl)amino)-L-phenylalanyl-4-(carbamoylamino)-D-phenylalanyl-L-leucyl-N6-(1-methylethyl)-L-lysyl-L-prolyl-;Degarelix acetate(FE-200486,Degarelix);FirMagon;Degarelix, Degarelix acetate | | CAS: | 214766-78-6 | | MF: | C82H103ClN18O16 | | MW: | 1632.26 | | EINECS: | 807-277-4 | | Product Categories: | API | | Mol File: | 214766-78-6.mol |  |
| | DEGARELIX Chemical Properties |
| density | 1.325±0.06 g/cm3(Predicted) | | storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C | | solubility | DMSO:10.0(Max Conc. mg/mL);6.13(Max Conc. mM)
H2O:25.0(Max Conc. mg/mL);15.32(Max Conc. mM) | | pka | 10.38±0.40(Predicted) | | InChIKey | MEUCPCLKGZSHTA-XYAYPHGZSA-N |
| | DEGARELIX Usage And Synthesis |
| Description | Antagonists of GnRH have proven to be an effective therapy for
hormonally regulated cancers, such as prostate and some types of
breast. As analogs of GnRH, they bind competitively and reversibly
to GnRH receptors in the pituitary gland, thereby blocking the release
of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In
men, the reduction of LH triggers the ablation of testosterone secretion
from the testes, and these castration-like levels have been essential in
the effective management of advanced prostate cancer. In comparison
to GnRH agonists, antagonists do not suffer from a potential flare of
the disease as a result of an initial stimulation of the hypothalamic-pituitary-gonadal axis prior to down-regulation of the GnRH
receptor. Moreover, GnRH antagonists provide beneficial effects more
rapidly postdosing and result in a more efficient suppression of gonadotropin levels.
With this in mind, degarelix acetate has been launched
as a third-generation GnRH antagonist for the treatment of prostate
cancer, and it joins other third-generation agents, ganirelix and cetronelix, on the market. | | Originator | Ferring Pharmaceutical (Switzerland) | | Uses | Degarelix, is a competitive and reversible gonadotropin-releasing hormone receptor (GnRHR) antagonist. | | Uses | Advanced hormone-dependent prostate carcinoma | | Definition | ChEBI: Degarelix is a polypeptide. | | Brand name | Firmagon | | Clinical Use | Ferring launched degarelix acetate, a gonadotrophin-releasing
hormone (GnRH) antagonist, in 2009 in the U.S. for the treatment
of prostate cancer. The compound has been approved by the
E.U. for the same indication, and in the same year it was launched
in the UK and Germany. Degarelix has been developed as a
one-month or three-month sustained-release injectable formulation.
Compared to other GnRH antagonists, degarelix displays
improved aqueous solubility, longer acting effects and weaker
histamine-releasing properties. | | Side effects | The most common adverse events included injection site reactions (pain, erythema, swelling, or induration), hot flashes, increased weight, and increases in serum levels of transaminases and gamma-glutamyltransferase. In addition to being contraindicated in patients with a previous hypersensitivity to degarelix, it should not be administered to women who are or may become pregnant as fetal harm can occur. Since long-term androgen deprivation therapy prolongs the QT interval, physicians should consider whether the benefits of degarelix outweigh the potential risks in patients with congenital long QT syndrome, electrolyte abnormalities, or congestive heart failure or in patients taking antiarrhythmic medications. | | Synthesis | The synthesis of degarelix acetate
employed iterative peptide coupling and protection/de-protection
sequences in high yields (85¨C99%), and this sequence is described
in the scheme. Boc-D-alanine (21) was immobilized via MBHA
resin (Bachem) by reaction with diisopropyl carbodiimide (DIC)
and 1-hydroxybenzotriazole (HOBT). The resulting product was
treated with trifluoroacetic acid (TFA) to remove the N-Boc
protecting group to reveal amine 22. The N-terminus of 22 was
then subjected to sequential coupling and de-protection cycles
with the following protected amino acids: N-Boc-L-proline, N-a-
Boc-N6-isopropyl-N6-carbobenzoxy-L-lysine and N-Boc-L-leucine
to give 23 and 24, respectively. The N-terminus of 24 was coupled
with N-a-Boc-D-4-(Fmoc-amino)phenylalanine, followed by removal
of the Fmoc group with piperidine in DMF to give the
corresponding free aniline. The free aniline resin was then reacted
with t-butyl isocyanate to generate the corresponding t-butyl urea followed by reaction with TFA to remove the Boc group to give the
t-butyl urea amine 25. The N-terminus of 25 was coupled with
N-a-Boc-L-4-(Fmoc-amino)phenylalanine, followed by removal of
the Fmoc group with piperidine in DMF to generate the corresponding
free aniline. The free aniline was reacted with L-hydroorotic
acid, followed by reaction with TFA to liberate amine 26.
Amine 26 was then coupled with O-benzylated-N-Boc-serine,
followed by removal of the Boc group with TFA and reacting the
resulting amine with N-a-Boc-D-(3-pyridyl)alanine and subsequent
removal of the Boc group with TFA gave amine 27. Amine
27 was coupled with N-Boc-D-(4-chlorophenyl)alanine, followed
by removal of the Boc group with TFA, and the resulting amine
was then coupled with N-Boc-D-(2-naphthyl)alanine, followed by
removal of its Boc group with TFA to give 28. Acylation of 28 with
acetic anhydride followed by sequential treatment with HF and
TFA resulted in cleavage from the resin, removal of the O-benzyl
group, and conversion of the t-butyl urea to the corresponding
NH2-urea, resulting in free degarelix. Finally, treatment with acetic
acid provided degarelix acetate (V). 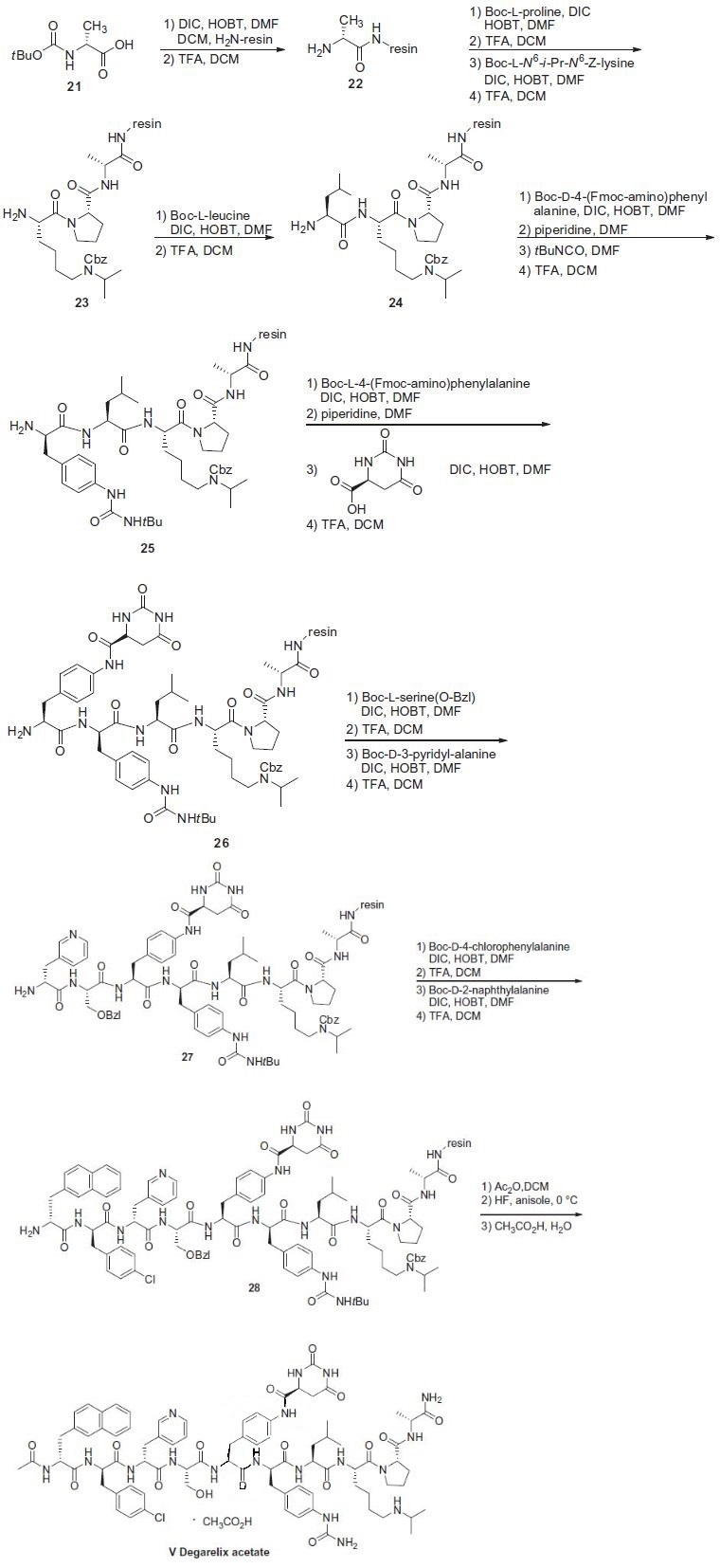
| | Metabolism | Undergoes peptide hydrolysis in the hepato-biliary
system, and is mainly (70-80%) excreted as peptide
fragments in the faeces. |
| | DEGARELIX Preparation Products And Raw materials |
|