|
| Product Name: | Ranolazine | | Synonyms: | (142387-99-3) ranolazine;1- 3-(2-Methoxyphenoxy)-2-hydroxypropyl -4- ...;1-Piperazineacetamide,N-(2,6-dimethylphenyl)-4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]-;N-(2,6-dimethylphenyl)-2-[4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]piperazin-1-yl]ethanamide;N-(2,6-diMethylphenyl)-2-{4-[(2R)-2-hydroxy-3-(2-Methoxyphenoxy)propyl]piperazin-1-yl}acetaMide;Ranolazine(Ranexa);100MG/500MG/1KG;Ranolazine
N-(2,6-Dimethylphenyl)-2-[4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]piperazin-1-yl]acetamide | | CAS: | 95635-55-5 | | MF: | C24H33N3O4 | | MW: | 427.54 | | EINECS: | 620-450-7 | | Product Categories: | Aromatics;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;RANEXA;Inhibitors;APIs;Antianginal;API | | Mol File: | 95635-55-5.mol |  |
| | Ranolazine Chemical Properties |
| Melting point | 119-1200C | | Boiling point | 624.1±55.0 °C(Predicted) | | density | 1.174±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | solubility | DMSO (Slightly), Methanol (Slightly) | | pka | 14.06±0.20(Predicted) | | form | Solid | | color | White | | CAS DataBase Reference | 95635-55-5(CAS DataBase Reference) |
| | Ranolazine Usage And Synthesis |
| Description | Ranolazine ([(+)N-(2,6-dimethylphenyl)-4(2-hydroxy-3-(2-methoxyphenoxy)-propyl)-1-piperazine acetamide dihydrochloride]) is an active piperazine derivative that was patented in 1986 and is available in an oral and intravenous form. Ranolazine is evidenced with anti-ischemic/antianginal properties in patients with chronic angina without clinically significant changes in heart rate or blood pressure.
Ranolazine is used for the treatment of angina (chronic chest pain). Researches show it also has potential use in cardiovascular conditions such as heart failure, acute and chronic myocardial ischemia, certain types of cardiac sodium channel gene mutations, and ventricular and supraventricular arrhythmias.
| | References | [1] Bernard R. Chaitman, Ranolazine for the Treatment of Chronic Angina and Potential Use in Other Cardiovascular Conditions, New Drugs and Technologies, 2006, vol. 113, 2462-2472
[2] Bernard R. Chaitman, Sandra L. Skettino, John O. Parker, Peter Hanley, Jaroslav Meluzin, Jerzy Kuch and Carl J. Pepine, Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina, Journal of the American College of Cardiology, 2004, vol. 43, 1375-1382
| | Description | Ranolazine is an orally available, extended release drug for the treatment of
chronic angina in patients who have failed to respond to prior angina therapy.
Chronic stable angina (CSA) is a common symptom of coronary artery disease
wherein plaques in the coronary vasculature restrict blood flow to the heart,
which in turn leads to insufficient oxygenation of the heart, typically during
physical exertion or emotional stress. A vast majority of the existing anti-anginal
and anti-ischemic therapies aim to correct the imbalance between myocardial
oxygen demand and supply through mechanisms that produce reductions in
heart rate or blood pressure. | | Chemical Properties | White Solid | | Originator | Roche Bioscience (US) | | Uses | antianginal, antiischemic | | Definition | ChEBI: N-(2,6-dimethylphenyl)-2-{4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]piperazin-1-yl}acetamide is an aromatic amide obtained by formal condensation of the carboxy group of 2-{4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]piperazin-1-yl}acetic acid with the amino group of 2,6-dimethylaniline. It is a monocarboxylic acid amide, an aromatic amide, a N-alkylpiperazine, a secondary alcohol and a monomethoxybenzene. | | Brand name | Ranexa (Sensus). | | General Description | Ranolazine, N-(2,6-dimethylphenyl)-2-[4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]piperazin-1-yl]acetamide (Ranexa), is an antianginal medication thatwas approved by the Food and Drug Administration (FDA)in January 2006 for the treatment of chronic angina.Ranolazine is believed to elicit its effects by altering thetranscellular late sodium current. This, in turn, alters thesodium-dependent calcium channels during myocardial ischemia.Thus, ranolazine indirectly prevents the calciumoverload that is associated with cardiac ischemia.Ranolazine is metabolized by the cytochrome CYP3A enzymesin the liver. | | Clinical Use | Add on therapy for angina | | Synthesis | Two syntheses,
one from the inventors at Roche and other from a group in Hungary, of Ranolazine have been described in the
patent literature. The original synthesis is highlighted in the
Scheme. Reaction of 2,6-dimethylaniline 46 with chloroacetyl
chloride (47) in the presence of triethylamine for 4h at
0oC gave amide 48 in 82% yield. This chloro amide 48 was
reacted with piperazine in refluxing ethanol for 2 h to give
piperazinyl amide 50. Reaction of amide 50 with epoxide
intermediate 53, prepared by reacting 2-methoxy phenol 51
with epichlorohydrin, in refluxing isopropanol for 3 h followed
by treatment with HCl/methanol gave ranolazine dihydrochloride
(VII) in 73% yield. 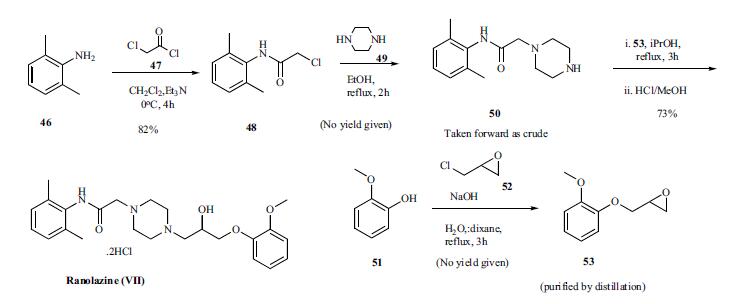
| | Drug interactions | Potentially hazardous interactions with other drugs
Anti-arrhythmics: avoid with disopyramide.
Antibacterials: concentration possibly increased
by clarithromycin and telithromycin - avoid
concomitant use; concentration reduced by
rifampicin - avoid.
Antifungals: concentration increased by ketoconazole
and possibly itraconazole, posaconazole and
voriconazole - avoid.
Antivirals: concentration possibly increased by
atazanavir, darunavir, fosamprenavir, indinavir,
lopinavir, ritonavir, saquinavir and tipranavir - avoid.
Beta-blockers: avoid with sotalol.
Ciclosporin: concentration of both drugs possibly
increased.
Grapefruit juice: concentration of ranolazine possibly
increased - avoid.
Statins: concentration of simvastatin increased -
maximum dose of simvastatin is 20 mg.
Tacrolimus: concentration of tacrolimus increased. | | Metabolism | Extensively metabolised in the gastrointestinal tract and
liver. Four main metabolites have been identified.
Approximately 75% of a dose is excreted in the urine with
the remainder in the faeces. |
| | Ranolazine Preparation Products And Raw materials |
|



