|
| | Azacyclotridecan-2-one Basic information |
| | Azacyclotridecan-2-one Chemical Properties |
| Melting point | 150-153 °C(lit.) | | Boiling point | 334.44°C (rough estimate) | | density | 0.9415 (rough estimate) | | vapor pressure | 0.001Pa at 20℃ | | refractive index | 1.4400 (estimate) | | Fp | 195 °C | | form | powder to crystal | | pka | 16.91±0.20(Predicted) | | color | White to Almost white | | Water Solubility | 223mg/L at 20℃ | | BRN | 122031 | | LogP | 2.71 at 20℃ | | EPA Substance Registry System | Azacyclotridecan-2-one (947-04-6) |
| | Azacyclotridecan-2-one Usage And Synthesis |
| Chemical Properties | Dry PowderSolid; very faintly beige crystalline lumps. Virtually insol in water. | | Preparation | One route to dodecyllactam is from butadiene as follows:
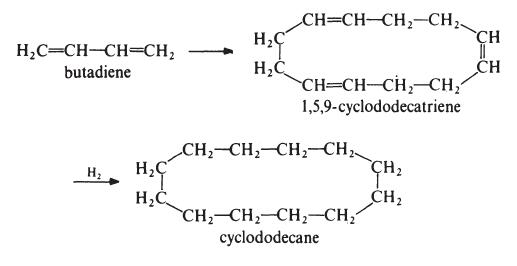
Butadiene is trimerized with a Ziegler-Natta catalyst to give 1,5,9-cyclododecatriene
which is then hydrogenated to cyclododecane. Dodecyllactam
is then obtained by a series of reactions similar to those used to prepare
caprolactam from cyclohexane. | | Definition | ChEBI: Laurolactam is an azamacrocycle and a keratan 6'-sulfate. | | Health Hazard | The acute oral LD50 was 2,330 mg/kg bw (rat) with central nervous system stimulation (trembling, convulsive twitches, ataxia) as the main clinical sign appearing at about 1,580 mg/kg bw. The dermal LD50 was greater than 2,000 mg/kg bw (rat). Decreased food consumption and stagnation of body weight development were noted. Validacute inhalation studies are not available. Azacyclotridecan-2-one was not irritating to the rabbit skin or eye (OECD TG 404, 405). It was not sensitizing in aguinea pig maximization test (OECD TG 406; 1981). | | Purification Methods | Azacyclotridecan-2-one crystallises from CHCl3 and is stored over P2O5 in a vacuum desiccator. [Beilstein 26/1 V 566.] |
| | Azacyclotridecan-2-one Preparation Products And Raw materials |
|



