|
| | POLY(METHYL METHACRYLATE) Basic information |
| | POLY(METHYL METHACRYLATE) Chemical Properties |
| Hazard Codes | Xn | | Risk Statements | 20/22 | | Safety Statements | 36 | | WGK Germany | 3 | | RTECS | TR0400000 | | Autoignition Temperature | 580 °F |
| | POLY(METHYL METHACRYLATE) Usage And Synthesis |
| Uses | Poly(methyl methacrylate-co-ethyl acrylate) (PMMAEA) copolymers may be used to prepare single phase mixture with phenolic derivative. PMMAEA may be used to form latex interpenetrating polymer networks (LIPNs). Membranes of PMMAEA were reportedly used for separation of heavy metal ions from aqueous solutions. | | Preparation | Acrylic acid is H2C=CH-COOH and methacrylic acid is H2C=C(CH3)COOH. These compounds and their methyl esters are both quite reactive and difficult to store and handle. The monomer used to form poly(methyl methacrylate), 2-hydroxy-2-methylpropanenitrile, is prepared by the following reaction:
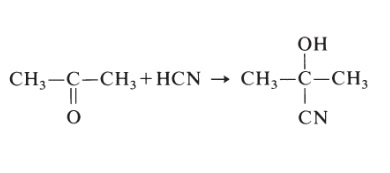
2-Hydroxy-2-methylpropanenitrile is then reacted with methanol (or other alcohol) to yield methacrylate ester. Free-radical polymerization is initiated by peroxide or azo catalysts and produce poly(methyl methacrylate) resins having the following formula:
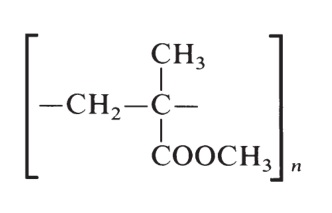
Key properties are good resistance to heat, light, and weathering. This polymer is unaffected by most detergents, cleaning agents, and solutions of inorganic acids, alkalies, and aliphatic hydrocarbons. Poly(methyl methacrylate) has light transmittance of 92% with a haze of 1–3% and its clarity is equal to glass. | | Industrial uses | Poly(methyl methacrylate), the polymerizedmethyl ester of methacrylic acid, is thermoplastic.The method of polymerization may be variedto achieve specific physical properties, orthe monomer may be combined with other components.Sheet materials may be prepared bycasting the monomer in bulk. Suspension polymerizationof the monomeric ester may be usedto prepare molding powders.
Conventional poly(methyl methacrylate) isamorphous; however, reports have been publishedof methyl methacrylate polymers of regularconfiguration, which are susceptible tocrystallization. Both the amorphous and crystallineforms of such crystallization-susceptible polymers possess physical properties that aredifferent from those of the conventional polymer,and suggest new applications. | | Industrial uses | Polymethyl methacrylate is a clear solid, hardbut easily scratched. Its dielectric properties areonly moderate, and its use is restricted to undemandingconditions at normal ambient temperatures,usually in a molded form, where appearanceis important. |
| | POLY(METHYL METHACRYLATE) Preparation Products And Raw materials |
|