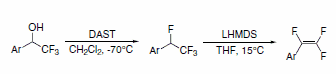|
| | Diethylaminosulfur trifluoride Chemical Properties |
| Boiling point | 30-32 °C3 mm Hg(lit.) | | density | 1.22 g/mL at 25 °C(lit.) | | refractive index | 1.41-1.416 | | Fp | 75 °F | | storage temp. | 2-8°C | | solubility | Benzene, Chloroform (Slightly) | | pka | -4.87±0.70(Predicted) | | form | liquid | | Specific Gravity | 1.220 | | color | Colorless to Yellow to Orange | | Water Solubility | decomposes | | Sensitive | Moisture Sensitive | | Merck | 14,3113 | | BRN | 1849066 | | Exposure limits | ACGIH: TWA 2.5 mg/m3
NIOSH: IDLH 250 mg/m3 | | Stability: | Reacts with Moisture in the Air, Reacts With Moisture In The Air, Unstable in So | | InChIKey | CSJLBAMHHLJAAS-UHFFFAOYSA-N | | CAS DataBase Reference | 38078-09-0(CAS DataBase Reference) | | NIST Chemistry Reference | Diethylaminosulfur trifluoride(38078-09-0) |
| | Diethylaminosulfur trifluoride Usage And Synthesis |
| Fluorinating reagents | N,N-Diethylaminosulfur trifluoride (DAST) is the most widely used fluorinating reagent in this class. It has a similar reactivity to sulfur tetrafluoride, but has the advantage that it can be used in glass vessels at atmospheric pressure. Deoxofluorination reactions with N,N-diethylaminosulfur trifluoride are usually run neat or in anhydrous aprotic solvents, most frequently halogenated solvents, at temperatures of 0- 80 °C. Typically, a stoichiometric amount of the reagent is used for aldehydes and a small excess is used for ketones.

Diethylaminosulfur trifluoride is the most widely used member of this family of reagents, is a pale yellow liquid that reacts violently with water. DAST is readily obtained from commercial sources (e.g. Aldrich or Lancaster) and can also be prepared by the reaction of N,N-di-ethylaminotrimethylsilane with sulfur tetrafluoride, followed by distillation of the crude product under reduced pressure. DAST is known to be thermally unstable, undergoing either explosion or detonation when heated to > 9°ºC.
| | Preparation | This preparation of diethylaminosulfur trifluoride is an adaptation of a procedure first described by von Halasz and Glemser.The same procedure can also be used to prepare other dialkylaminosulfur trifluorides by the substitution of the diethylaminotrimethylsilane with other dialkylaminotrimethylsilanes.
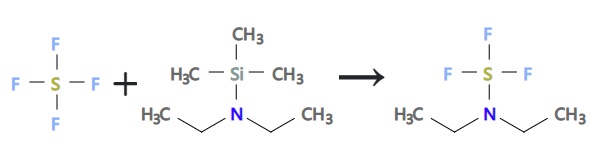
A solution of 96 g (0.66 mole) of diethylaminotrimethylsilane in 100 ml trichlorofluoromethane was added dropwise to a solution of 40 ml (measured at -78°, 0.72 mole) of sulfur tetrafluoride in 200 ml of trichlorofluoromethane at -65° to -60°. The reaction mixture was warmed to room temperature and then distilled to give 88.86 g (84% yield) of diethylaminosulfur trifluoride as a pale yellow liquid, bp 46°-47° (10 mm).
| | Reactions | Diethylaminosulfur trifluoride (DAST) has proven itself to be an extremely popular reagent for nucleophilic fluorination, due to its ease of handling and versatility. It has regularly been employed in a myriad selective fluorinations of alcohols, alkenols, carbohydrates, ketones, sulfides, epoxides, thioethers, and cyanohydrins. In addition some novel organic cyclizations are possible when DAST is employed as a reagent.
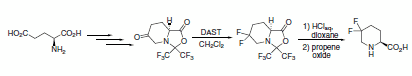
Fluorodeoxygenation was achieved using DAST in a preparatively simple synthesis of 5,5-difluoropipecolic acid from glutamic acid.
1,2,2-Trifluorostyrenes can be synthesized using a sequential reaction on the parent a-(trifluoromethyl)phenylethanol with DAST, followed by dehydrohalogenation with lithium bis(trimethylsilyl)amide (LHMDS). This method achieves the trifluorostyrene without requirement of palladium coupling.
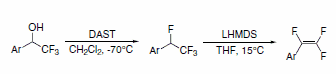
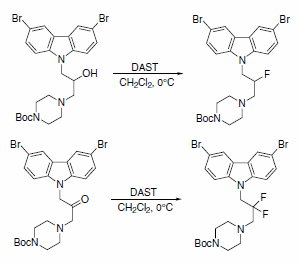
DAST was recently used to obtain fluorinated analogues of 3,6-dibromocarbazole piperazine derivatives of 2-propanol (Scheme 7). A series of these analogues are described as the first small and potent modulators of the cytochrome c release triggered by Bid-induced Bax activation in a mitochondrial assay.
| | Precautions | diethylaminosulfur trifluoride (DAST) reacts violently with water, it is volatile, and is reported to be explosive when heated owing to its calorimetry profile and its rapid dismutation to SF4 and (Et2N)2SF2. Furthermore, its decomposition products are themselves highly reactive and will etch laboratory glassware. Accordingly, DAST must be used with extreme caution, and samples are recommended not to be heated above 90 °C. | | Chemical Properties | Pale yellow liquid. Soluble in most non-polar organic solvents, commonly used in CH2Cl2, CHCl3 and CCl4. | | Uses | Diethylaminosulfur Trifluoride is a very useful Nucleophilic fluorinating agent in chemical synthesis. It is also used as a reagent in the preparation of 2-thiazolines from (1,2)-thioam ido-alcohols. | | Application | Diethylaminosulfur trifluoride (DAST) is used as a precursor to prepare 2,6-dimethoxybenzoyl fluoride and 2-thiazolines. It is a useful fluorinating agent used for a variety of compounds including alcohols, thioethers, alkenols and cyanohydrins. It also serves as a catalyst for Friedel-Crafts allylation reaction using tertiary cyclopropyl silyl ethers and in the rearrangement of homoallylic alcohols to unsaturated aldehydes. It plays an important role for gem difluorination of ketopipecolinic acids. |
| | Diethylaminosulfur trifluoride Preparation Products And Raw materials |
| Raw materials | Sodium hydroxide-->Acetone-->CARBON DIOXIDE-->Diethylamine-->Trimethylsilyl cyanide-->Trichlorofluoromethane-->Sulfur tetrafluoride-->N,N-Diethyl-1,1,1-trimethylsilylamine-->Triethylamine trihydrofluoride | | Preparation Products | 2-BENZYL-2,3-DIHYDROISOQUINOLIN-4(1H)-ONE HYDROCHLORIDE-->Amidosulfurous fluoride, N,N-diethyl--->Pentane, 1,1,2,2,3,3,4,4,5-nonafluoro- |
|