|
| | 2-Iodoaniline Basic information |
| | 2-Iodoaniline Chemical Properties |
| Melting point | 55-58 °C (lit.) | | Boiling point | 262.0±23.0 °C(Predicted) | | density | 1.8155 (estimate) | | Fp | >230 °F | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | solubility | soluble in Chloroform, DCM, Ethyl Acetate, Toluene | | form | Crystalline Needles | | pka | 2.6(at 25℃) | | color | Yellow to brown | | Water Solubility | Insoluble in water. | | Sensitive | Light Sensitive | | BRN | 2204899 | | InChIKey | UBPDKIDWEADHPP-UHFFFAOYSA-N | | CAS DataBase Reference | 615-43-0(CAS DataBase Reference) | | NIST Chemistry Reference | Benzenamine, 2-iodo-(615-43-0) | | EPA Substance Registry System | o-Iodoaniline (615-43-0) |
| | 2-Iodoaniline Usage And Synthesis |
| Chemical Properties | YELLOW TO BROWN CRYSTALLINE NEEDLES | | Uses | 2-Iodobenzenamine is an synthetic intermediate for the synthesis of complex organic compounds and pharmaceuticals. | | Uses | 2-Iodoaniline is used as an intermediate in organic synthesis. | | Preparation | 2-iodoaniline and its derivatives are commonly used for the preparation of indole-based anticancer drugs, which have high market value, and further synthesis can lead to high-end synthetic intermediates with complex structures.
Take a reaction tube, add 50mg of sodium azide, 75mg of 1-(2-iodophenyl)ethanol, 300uL of trifluoroacetic acid, 150uL of methanesulfonic acid and 1.0mL of hexane, and stir for 24 hours at 40℃. After the reaction, 10 mL of sodium hydroxide solution was added to quench the reaction. The organic phase was washed with 5 mL of brine, and the organic phase was combined and separated by column chromatography to obtain 58.4 mg of 2-iodoaniline in 89% yield.
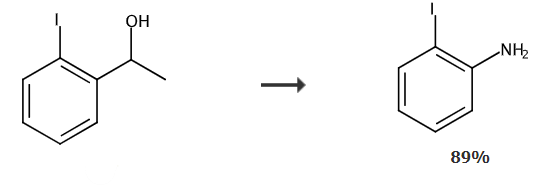
Synthesis of 2-iodoaniline | | Purification Methods | Distil 2-iodoaniline with steam and crystallise it from *benzene/pet ether. The N-acetyl derivative has m 110o. [Beilstein 12 IV 1542.] |
| | 2-Iodoaniline Preparation Products And Raw materials |
|



