| | Formic acid Chemical Properties |
| Melting point | 8.2-8.4 °C (lit.) | | Boiling point | 100-101 °C (lit.) | | density | 1.22 g/mL at 25 °C (lit.) | | vapor density | 1.03 (vs air) | | vapor pressure | 52 mm Hg ( 37 °C) | | refractive index | n20/D 1.377 | | FEMA | 2487 | FORMIC ACID | | Fp | 133 °F | | storage temp. | 2-8°C | | solubility | H2O: soluble1g/10 mL, clear, colorless | | pka | 3.75(at 20℃) | | form | Liquid | | Specific Gravity | 1.216 (20℃/20℃) | | color | APHA: ≤15 | | PH | 3.47(1 mM solution);2.91(10 mM solution);2.38(100 mM solution); | | Odor | at 0.10 % in water. pungent vinegar formyl | | Odor Type | acetic | | explosive limit | 12-38%(V) | | Water Solubility | MISCIBLE | | λmax | λ: 260 nm Amax: 0.03
λ: 280 nm Amax: 0.01 | | Sensitive | Hygroscopic | | Merck | 14,4241 | | JECFA Number | 79 | | BRN | 1209246 | | Henry's Law Constant | At 25 °C: 95.2, 75.1, 39.3, 10.7, and 3.17 at pH values of 1.35, 3.09, 4.05, 4.99, and 6.21,
respectively (Hakuta et al., 1977) | | Exposure limits | TLV-TWA 5 ppm (~9 mg/m3) (ACGIH,
MSHA, OSHA, and NIOSH); IDLH 100
ppm (180 mg/m3) (NIOSH). | | Stability: | Stable. Substances to be avoided include strong bases, strong oxidizing agents and powdered metals, furfuryl alcohol. Combustible. Hygroscopic. Pressure may build up in tightly closed bottles, so bottles should be opened carefully and vented periodically. | | InChIKey | BDAGIHXWWSANSR-UHFFFAOYSA-N | | LogP | -0.540 | | CAS DataBase Reference | 64-18-6(CAS DataBase Reference) | | NIST Chemistry Reference | Formic acid(64-18-6) | | EPA Substance Registry System | Formic acid (64-18-6) |
| | Formic acid Usage And Synthesis |
| General Description | Formic acid (HCO2H), also called methanoic acid, is the simplest carboxylic acid. Formic acid was first isolated by the distillation of ant bodies and was named after the Latin formica, meaning “ant.” Its proper IUPAC name is now methanoic acid. Industrially, formic acid is produced by treatment of carbon monoxide with an alcohol such as methanol (methyl alcohol) in the presence of a catalyst.
Formic acid is found both naturally occurring and frequently synthesized in laboratories. It is most naturally found in the stings and bites of many insects, including bees and ants, as a chemical defense mechanism.
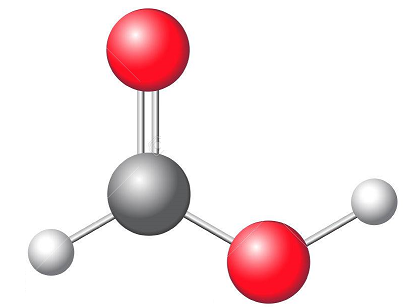
formic acid structure
| | Properties | FORMIC ACID is a colorless liquid with a pungent odor. It is a stable corrosive, combustible, and hygroscopic chemical substance. It is incompatible with H2SO4, strong caustics, furfuryl alcohol, hydrogen peroxide, strong oxidisers, and bases and reacts with strong explosion on contact with oxidising agents.
Due to the −CHO group, Formic acid imparts some of the character of an aldehyde. It can form salt and ester; can react with amine to form amide and to form ester by addition reaction with unsaturated hydrocarbon addition. It can reduce the silver ammonia solution to produce a silver mirror, and make the potassium permanganate solution fade, which can be used for the qualitative identification of formic acid.
As a carboxylic acid, formic acid shares most of the same chemical properties in reacting with alkalis to form water soluble formate. But formic acid is not a typical carboxylic acid as it can react with alkenes to form formate esters.
| | Production | - Since 1896, Formic acid is made in European countries by the action of sulfuric acid upon sodium formate, which is produced from carbon monoxide and sodium hydroxide.
 - In 1980, the United States Science and Design Corporation developed a carbonylation of methanol to produce formic acid with an annual output of 20,000 tons. The reaction formula is:
 - The mixture of liquid ammonia and methanol is used to absorb carbon monoxide at 70 ° C and 32.5 MPa to form formamide, which is then hydrolyzed in an aqueous acid solution.
- Use oxalic acid and glycerol as raw materials being co-heated at 110 ° C to generate oxalic acid monoglyceride. Heat it to decarboxylate and form Monoglycerides formate, then hydrolyze it to obtain formic acid.
- After the formic acid aqueous solution is obtained, a dehydrating agent (for anhydrous magnesium sulfate, anhydrous copper sulfate, etc.), extractive distillation (extracting agent may be trimethylamine, picoline, etc.) may be used for dehydration and purification, and anhydrous formic aic can be obtained.
| | Description | Formic acid is a clear, colorless liquid with a pungent odor.
Formic acid was first isolated from certain ants and was named
after the Latin formica, meaning ant. It is made by the action of
sulfuric acid on sodium formate, which is produced from
carbon monoxide and sodium hydroxide. It is also produced as
a by-product in the manufacture of other chemicals such as
acetic acid.
It can be anticipated that use of formic acid will continuously
increase as it replaces inorganic acids and has a potential
role in new energy technology. Formic acid toxicity is of
a special interest as the acid is the toxic metabolite of methanol. | | Chemical Properties | Formic acid, or methanoic acid, is the first member of the homologous series identified as fatty acids with the general formula RCOOH. Formic acid was obtained first from the red ant; itscommon name is derived from the family name for ants, Formicidae. This substance also occurs naturally in bees and wasps, and is presumed to be responsible for the "sting" of these insects. Formic acid has a pungent, penetrating odor. It may be synthesized from anhydrous sodium formate and concentrated H2S04 at low temperature followed by distillation. | | Physical properties | Clear, colorless, fuming liquid with a pungent, penetrating odor. Odor threshold concentration is 49 ppm (quoted, Amoore and Hautala, 1983). it is miscible in water, alcohol, ether, and glycerin, and is obtained by chemical synthesis or oxidation of methanol or formaldehyde. | | Occurrence | Widespread in a large variety of plants; reported identifed in Cistus labdanum and the oil of Artemisia trans- iliensis; also found among the constituents of petit grain lemon and bitter orange essential oil; reported found in strawberry aroma Reported found in apple, sweet cherry, papaya, pear, raspberry, strawberry, peas, cheeses, breads, yogurt, milk, cream, buttermilk, raw fsh, cognac, rum, whiskey, cider, white wine, tea, coffee and roasted chicory root | | History | Formic acid is taken from the Latin word forant, formica. Naturalists had observed
the acrid vapor from ant hills for hundreds of years. One of the earliest descriptions of formic
acid was reported in an extract of a letter written from John Wray (1627–1705) to the publisher
of Philosophical Transactions published in 1670. Wray’s letter reported on “uncommon
Observations and Experiments made with an Acid Juyce to be Found in Ants” and noted the
acid was previously obtained by Samuel Fisher from the dry distillation of wood ants. Formic
acid is found in stinging insects, plants, unripe fruit, foods, and muscle tissue. J?ns Jacob
Berzelius (1779–1848) characterized formic acid in the early 19th century, and it wasfirst synthesized
from hydrocyanic acid by Joseph Louis Gay-Lussac (1778–1850) at about the same
time. A number of synthetic preparations of formic acid were found in the first half of the
19th century. Marcellin Berthelot (1827–1907) discovered a popular synthesis using oxalic
acid and glycerin in 1856; he and several other chemists from his period found syntheses of
formic acid by heating carbon monoxide in alkaline solutions. | | Uses | Formic acid has a number of commercial uses. It is used in the leather industry to degreaseand remove hair from hides and as an ingredient in tanning formulations. It is used as alatex coagulant in natural rubber production. Formic acid and its formulations are used aspreservatives of silage. It is especially valued in Europe where laws require the use of naturalantibacterial agents rather than synthetic antibiotics. Silage is fermented grass and crops thatare stored in silos and used for winter feed. Silage is produced during anaerobic fermentationwhen bacteria produce acids that lower the pH, preventing further bacterial action. Acetic acidand lactic acid are the desired acids during silage fermentation. Formic acid is used in silageprocessing to reduce undesirable bacteria and mold growth. Formic acid reduces Clostridiabacteria that would produce butyric acid causing spoilage. In addition to preventing silagespoilage, formic acid helps preserve protein content, improves compaction, and preservessugar content. Formic acid is used as a miticide by beekeepers. | | Uses | Formic acid occurs in the stings of ants andbees. It is used in the manufacture of estersand salts, dyeing and finishing of textiles andpapers, electroplating, treatment of leather,and coagulating rubber latex, and also as areducing agent. | | Definition | ChEBI: Formic acid is the simplest carboxylic acid, containing a single carbon. Occurs naturally in various sources including the venom of bee and ant stings, and is a useful organic synthetic reagent. Principally used as a preservative and antibacterial agent in livestock feed. Induces severe metabolic acidosis and ocular injury in human subjects. It has a role as an antibacterial agent, a protic solvent, a metabolite, a solvent and an astringent. It is a conjugate acid of a formate. | | Production Methods | Formic acid is manufactured as a by-product of the liquidphase
oxidation of hydrocarbons to acetic acid. It is
also produced by (a) treating sodium formate and
sodium acid formate with sulfuric acid at low temperatures
followed by distillation or (b) direct synthesis
from water and CO2 under pressure and in the presence of
catalysts. | | Reactions | Formic acid solution reacts as follows: (1) with hydroxides, oxides, carbonates, to form formates, e.g., sodium formate, calcium formate, and with alcohols to form esters; (2) with silver of ammonio-silver nitrate to form metallic silver; (3) with ferric formate solution, upon heating, to form red precipitate of basic ferric formate; (4) with mercuric chloride solution to form mercurous chloride, white precipitate; and (5) with permanganate (in the presence of dilute H2SO4) to form CO2 and manganous salt solution. Formic acid causes painful wounds when it comes in contact with the skin. At 160 °C, formic acid yields CO2 plus H2. When sodium formate is heated in vacuum at 300 °C, H2 and sodium oxalate are formed. With concentrated H2SO4 heated, sodium formate, or other formate, or formic acid, yields carbon monoxide gas plus water. Sodium formate is made by heating NaOH and carbon monoxide under pressure at 210 °C. | | Biotechnological Production | Formic acid is generally produced by chemical synthesis . However, biotechnological
routes are described in literature. First, formic acid could be produced
from hydrogen and bicarbonate by whole-cell catalysis using a methanogen.
Concentrations up to 1.02 mol.L-1 (47 g.L-1) have been reached within 50 h. Another example is the formation of formic acid and ethanol as co-products
by microbial fermentation of glycerol with genetically modified organisms. In
small-scale experiments, 10 g.L-1 glycerol has been converted to 4.8 g.L-1 formate
with a volumetric productivity of 3.18 mmol.L-1.h-1 and a yield of 0.92 mol
formate per mole glycerol using an engineered E. coli strain. | | Taste threshold values | Taste characteristics at 30 ppm: acidic, sour and astringent with a fruity depth. | | General Description | Formic acid is the simplest carboxylic acid. Crystal structure study by single-crystal X-ray diffraction technique at -50°C has shown that it has an orthorhombic structure with space group Pna. The photodegradation of formic acid has been investigated using ab intio calculations and time-resolved Fourier transform infrared spectroscopy. Its utility as a fuel in direct fuel cells has been studied. The momentum distribution for its monomer have been evaluated by electron momentum spectroscopy (EMS). | | Air & Water Reactions | Fumes in air. Soluble in water with release of heat. | | Reactivity Profile | Formic acid reacts exothmerically with all bases, both organic (for example, the amines) and inorganic. Reacts with active metals to form gaseous hydrogen and a metal salt. Reacts with cyanide salts to generate gaseous hydrogen cyanide. Reacts with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides to generate flammable or toxic gases. Reacts with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Reacts with carbonates and bicarbonates to generate carbon dioxide but still heat. Can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. May initiate polymerization reactions or catalyze other chemical reactions. A mixture with furfuryl alcohol exploded [Chem. Eng. News 18:72(1940)]. | | Hazard | Corrosive to skin and tissue. | | Health Hazard | Formic acid is a low to moderately toxicbut highly caustic compound. It is corrosiveto the skin, and contact with pure liquidcan cause burns on the skin and eyes. It ismore toxic than acetic acid. Formic acid isa metabolite of methanol responsible for thelatter’s toxicity. Thus, the acute acidosis ofmethanol is due to the in vivo formation offormic acid generated by the actionof enzymes, alcohol dehydrogenase, andaldehyde dehydrogenase. Ingestion of formicacid can cause death. Long-term exposure toformic acid can cause kidney damage. Greenet al. (2003) have found increased excretionof formic acid and the development of kidneytoxicity in rats following chronic dosingwith trichloroethanol and trichloroethylene.Such induced nephrotoxicity was attributedto excretion and acidosis from formic acid.
Exposure to formic acid vapors may produce irritation of the eyes, skin, and mucousmembranes, causing respiratory distress.
LD50 value, oral (mice): 700 mg/kg
LC50 value, inhalation (mice): 6200 mg/m3/15 minutes
Liesivuori and Savolainen (1991) studiedthe biochemical mechanisms of toxicityof methanol and formic acid. Formicacid is an inhibitor of the enzymemitochondrial cytochrome oxidase causinghistotoxic hypoxia. It is, however, a weakerinhibitor than cyanide and hydrosulfideanions. The effects of its acidosis are dilationof cerebral vessels, facilitation of the entryof calcium ions into cells, loss of lysosomallatency, and deranged production of ATP, thelatter affecting calcium reabsorption in thekidney tubules. Also, urinary acidificationfrom formic acid and its excretion maycause continuous recycling of the acid bythe tubular cell Cl-/formate exchanger. Suchsequence of events probably causes anaccumulation of formate in urine. Other thanmethanol, methyl ethers, esters, and amidesalso metabolize forming formic acid
Chan et al. (1995) have reported a caseof systemic toxicity developed in a 3-yearold girl burned by formic acid over 35% of her total body surface area. The metabolicacidosis in this case was profound with theserum formate level reaching at 400 μg/mL,the highest reported in the literature forpoisoning by any route. The patient wassuccessfully treated with hemodialysis, IVbicarbonate, and supportive measures.
In a study on the poisoning effect ofmethanol and its toxic metabolite formicacid on the retinal photoreceptors and theretinal pigment epithelian cells Treichel et al.(2004) found that the cytotoxic effects weregreater in the retinal photoreceptors althoughboth the cell types accumulated similar levelsof formate when their cultured cell lineswere exposed to formic acid in vitro. Formicacid treatment in both cell types produceddecreases in glutathione and glutathioneperoxidase. | | Fire Hazard | Special Hazards of Combustion Products: Toxic vapor generated in fires | | Safety Profile | Poison by inhalation,
intravenous, and intraperitoneal routes.
Moderately toxic by ingestion. Mutation data
reported. Corrosive. A skin and severe eye
irritant. A substance migrating to food from packaging materials. Combustible liquid
when exposed to heat or flame; can react
vigorously with oxidizing materials.
Explosive reaction with furfuryl alcohol,
H202, T1(NO3)3*3H2O nitromethane, P2O5.
To fight fire, use CO2, dry chemical, alcohol
foam. When heated to decomposition it
emits acrid smoke and irritating fumes. | | Potential Exposure | Formic acid is a strong reducing agent
and is used as a decalcifier. It is used in pharmaceuticals;
in dyeing textiles and finishing color-fast wool; electroplat ing, coagulating latex rubber; regeneration old rubber, and
dehairing, plumping, and tanning leather. It is also used in
the manufacture of acetic acid, airplane dope; allyl alcohol;
cellulose formate; phenolic resins; and oxalate; and it is
used in the laundry, textile, insecticide, refrigeration, and
paper industries; as well as in drug manufacture. | | Source | Formic acid naturally occurs in carrots, soybean roots, carob, yarrow, aloe, Levant
berries, bearberries, wormwood, ylang-ylang, celandine, jimsonweed, water mint, apples,
tomatoes, bay leaves, common juniper, ginkgo, scented boronia, corn mint, European pennyroyal,
and bananas (Duke, 1992).
Formic acid was formed when acetaldehyde in the presence of oxygen was subjected to
continuous irradiation (λ >2200 ?) at room temperature (Johnston and Heicklen, 1964).
Formic acid was identified as a constituent in a variety of composted organic wastes. Detectable
concentrations were reported in 16 of 21 composts extracted with water. Concentrations ranged
from 0.02 mmol/kg in a sawdust + dairy cattle manure to 30.65 mmol/kg in fresh dairy manure.
The overall average concentration was 9.64 mmol/kg (Baziramakenga and Simard, 1998). | | Environmental fate | Biological. Near Wilmington, NC, organic wastes containing formic acid (representing 11.4%
of total dissolved organic carbon) were injected into an aquifer containing saline water to a depth
of about 1,000 feet. The generation of gaseous components (hydrogen, nitrogen, hydrogen sulfide,
carbon dioxide, and methane) suggested that formic acid and possibly other waste constituents
were anaerobically degraded by microorganisms (Leenheer et al., 1976).
Heukelekian and Rand (1955) reported a 5-d BOD value of 0.20 g/g which is 57.1% of the
ThOD value of 0.83 g/g.
Photolytic. Experimentally determined rate constants for the reaction of formic acid with OH
radicals in the atmosphere and aqueous solution were 3.7 x 10-13 and 2.2 x 10-13 cm3/molecule?
sec, respectively (Dagaut et al., 1988).
Chemical/Physical. Slowly decomposes to carbon monoxide and water. At 20 °C, 0.06 g of
water would form in 1 yr by 122 g formic acid. At standard temperature and pressure, this amount
of formic acid would produce carbon monoxide at a rate of 0.15 mL/h. The rate of decomposition
decreases with time because the water produced acts as a negative catalyst (Barham and Clark,
1951).
Slowly reacts with alcohols and anhydrides forming formate esters.
At an influent concentration of 1.00 g/L, treatment with GAC resulted in an effluent concentration
of 765 mg/L. The adsorbability of the GAC used was 47 mg/g carbon (Guisti et al., 1974). | | Shipping | UN1779 Formic acid, with>85% acid by mass,
Hazard class: 8; Labels: 8-Corrosive material, 3-Flammable
liquid | | Purification Methods | Anhydrous formic acid can be obtained by direct fractional distillation under reduced pressure, the receiver being cooled in ice-water. The use of P2O5 or CaCl2 as dehydrating agents is unsatisfactory. Reagent grade 88% formic acid can be satisfactorily dried by refluxing with phthalic anhydride for 6hours and then distilling it. Alternatively, if it is left in contact with freshly prepared anhydrous CuSO4 for several days about one half of the water is removed from 88% formic acid; distillation then removes the remainder. Boric anhydride (prepared by melting boric acid in an oven at a high temperature, cooling in a desiccator, and powdering) is a suitable dehydrating agent for 98% formic acid; after prolonged stirring with the anhydride the formic acid is distilled under vacuum. Formic acid can be further purified by fractional crystallisation using partial freezing. [Beilstein 2 IV 3.] | | Toxicity evaluation | Formic acid toxicity is based on the inhibitory capability of the
cytochrome oxidase, a terminal member of the eukaryotic
mitochondrial electron transport chain and an integral protein
complex of the inner mitochondrial membrane. This enzyme
participates in the four-electron reduction of oxygen molecule
to water with concomitant synthesis of ATP. Formic acid
inhibits the activity of cytochrome oxidase by binding at the
sixth coordination position of ferric heme iron. The cytochrome
oxidase inhibition by formic acid increases with
decreasing pH, suggesting that the active inhibitor is the
undissociated acid. The acid is permeable through the inner
mitochondrial membrane only in this form. Acidosis may
potentiate the inhibition of cellular respiration and hasten the
onset of cellular injury. Also the progressive acidosis will
induce circulatory failure. This leads to tissue hypoxia and lactic
acid production, both of which further increase the acid load,
in turn increasing undissociated formic acid. This cycle is
termed ‘circulus hypoxicus.’
The acidosis causes, e.g., dilatation of cerebral vessels,
facilitation of the entry of calcium ions into cells, loss of
lysosomal latency, and deranged production of ATP. The last
effect seems to impede parathormone-dependent calcium
reabsorption in the kidney tubules. Besides, urinary acidification
is affected by formic acid. Its excretion causes continuous
recycling of the acid by the tubular cell chloride/formate
exchanger, which may partially explain an accumulation of
formate in urine. | | Incompatibilities | Vapors may form explosive mixture with
air. A medium strong acid and a strong reducing agent.
Violent reaction with oxidizers, furfuryl alcohol; hydrogen
peroxide; nitromethane. Incompatible with strong acids;
bases, ammonia, aliphatic amines; alkanolamines, isocya nates, alkylene oxides; epichlorohydrin. Decomposes on
heating and on contact with strong acids forming carbon
monoxide. Carbamates are incompatible with strong acids
and bases, and especially incompatible with strong reducing
agents such as hydrideds and active metals. Contact with
active metals or nitrides form flammable gaseous hydrogen.
Incompatible with strongly oxidizing acids, peroxides, and
hydroperoxides. Attacks metals: aluminum, cast iron and
steel; many plastics, rubber and coatings. | | Waste Disposal | Incineration with added
solvent. Consult with environmental regulatory agencies
for guidance on acceptable disposal practices. Generators
of waste containing this contaminant (≥ kg/mo) must
conform with EPA regulations governing storage, transpor tation, treatment, and waste disposal. |
| | Formic acid Preparation Products And Raw materials |
| Raw materials | Sodium hydroxide-->Methanol-->Sulfuric acid-->Triethylamine-->Ammonia-->Sodium Methoxide-->Phosphoric acid-->CARBON MONOXIDE-->PETROLEUM ETHER-->Sodium formate-->Methyl formate-->METALLURGICAL COKE | | Preparation Products | [4-AMINO-2-(TRIFLUOROMETHYL)PYRIMIDIN-5-YL]METHANOL-->8-BROMO-3-METHYL-3,7-DIHYDRO-PURINE-2,6-DIONE-->Sulfur Yellow GC-->2-Ethylbutyric acid-->3,4-DIHYDROISOQUINOLINE-->Benzyl formate-->N-Methylformamide-->1-METHYLPIPERIDINE-4-CARBOXYLIC ACID HYDROCHLORIDE-->2-METHANESULFONYL-4-METHYL-PYRIMIDINE-->5-Ethyl-2-pyridineethanol-->METHYL PENT-4-YN-2-YLCARBAMATE-->Sulfur Red Brown B3R-->Rhodinol-->5-Methyl-2-(methylsulfonyl)pyrimidine ,97%-->5-AMINO-6-CHLORO-PYRIMIDIN-4-OL-->4-AMINO-2-(TRIFLUOROMETHYL)PYRIMIDINE-5-CARBALDEHYDE-->7-Hydroxy-6-methoxy-3,4-dihydroisoquinoline-->1-METHYL-1-CYCLOHEXANECARBOXYLIC ACID-->(1H-INDAZOL-3-YL)-ACETIC ACID-->Diethoxymethyl acetate-->Epoxidized soya bean oil-->C.I.BASICBLUE22-->dimethyl dodecyl thioic propylene betaine-->5,7-dichlorooxazolo[5,4-d]pyrimidine-->GUANINE SULFATE-->1-FORMYL-4-METHYLPIPERAZINE-->4-Azabenzimidazole-->1,3,5-TRIMETHYL-1,3,5-TRIPHENYLCYCLOTRISILOXANE-->4,4'-Methylenebis(N,N-dimethylaniline)-->3-FLUORO-N-METHYLANILINE-->2-Hydroxy-N-(4-methoxyphenyl)-11H-benzo[a]carbazole-3-carboxamide-->Pigment Yellow 83-->2-AMINO-4,4'-DICHLORODIPHENYL ETHER-->2-OXO-2,3-DIHYDRO-BENZOOXAZOLE-5-CARBOXYLIC ACID-->1-(2-Hydroxyethyl)-4-methylpiperazine-->1H-Benzimidazole-5-carboxylic acid-->3-METHYLTHIOPHENE-2-CARBONITRILE-->Geranyl formate-->ETHYL 2,4-DICHLOROBENZOATE-->CINNAMYL FORMATE |
|





