|
| | Chlorotrifluoroethylene Basic information |
| | Chlorotrifluoroethylene Chemical Properties |
| | Chlorotrifluoroethylene Usage And Synthesis |
| Chemical Properties | Colorless gas; faint ethereal odor.
Decomposes in water. | | Uses | Intermediate, monomer for chlorotrifluoroethylene resins. | | Preparation | Chlorotrifluoroethylene is prepared from ethylene by the following route:
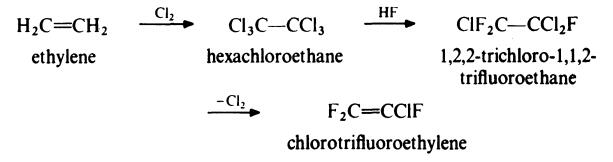
Ethylene is treated with an excess of chlorine at 300-350??C in the presence of
activated charcoal to give hexachloroethane. This product is then treated
with hydrogen fluoride in the presence of antimony pentachloride to yield
trichlorotrifluoroethane. Dechlorination in the liquid phase with zinc dust
and ethanol results in the formation of chlorotrifluoroethylene which is
washed with water to remove alcohol, dried and distilled under pressure. | | Synthesis Reference(s) | The Journal of Organic Chemistry, 57, p. 5018, 1992 DOI: 10.1021/jo00044a044 | | General Description | Chlorotrifluoroethylene is a colorless gas with a faint ethereal odor. Chlorotrifluoroethylene is shipped as a liquefied gas under its vapor pressure. Chlorotrifluoroethylene is very toxic by inhalation and is easily ignited. The vapors are heavier than air and a flame can flash back to the source of leak very easily. This leak can either be a liquid or vapor leak. The vapors can asphyxiate by the displacement of air. Contact with the liquid can cause frostbite. Under prolonged exposure to intense heat or fire the containers may violently rupture and rocket, or the material may polymerize with possible container rupture. | | Air & Water Reactions | Highly flammable. | | Reactivity Profile | Halogenated aliphatic compounds, such as Chlorotrifluoroethylene, are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Low molecular weight haloalkanes are highly flammable and can react with some metals to form dangerous products. Materials in this group are incompatible with strong oxidizing and reducing agents. Also, they are incompatible with many amines; nitrides; azo/diazo compounds; alkali metals; strong oxidizers such as chlorine perchlorate, oxygen, bromine; and epoxides. | | Hazard | Dangerous fire risk. Flammable limits in
air 8.4–38.7%. | | Health Hazard | Inhalation causes dizziness, nausea, vomiting; liver and kidney injury may develop after several hours and cause jaundice and necrosis of the kidney. Contact with liquid causes frostbite of eyes and possibly of skin. | | Flammability and Explosibility | Extremelyflammable | | Safety Profile | Poison by ingestion and
intraperitoneal routes. Moderately toxic by
inhalation. Very dangerous fire hazard when
exposed to heat, flames (sparks), or
oxidizers. To fight fire, stop flow of gas.
Violent reaction when mixed with (Brz +
02) or (CIF3 + water). Potentially explosive
polymerization reaction with ethylene.
Incompatible with 1,l -dchloroethylene;
oxygen. When heated to decomposition it
emits toxic fumes of Fand Cl-. See also
CHLORINATED HYDROCARBONS,
ALIPHATIC; and FLUORIDES. | | Purification Methods | Scrub it with 10% KOH solution, then 10% H2SO4 solution to remove inhibitors, dry and pass it through silica gel. It is stabilized with ~1% tributylamine. Use brass equipment. [Beilstein 1 III 646.] TOXIC GAS. |
| | Chlorotrifluoroethylene Preparation Products And Raw materials |
| Raw materials | ZINC-->Zinc chloride-->Sodium thiosulfate pentahydrate-->1,1,2-Trichlorotrifluoroethane-->Silane, trichloro(2,2-dichloro-1,1,2-trifluoroethyl)--->1-bromo-1,2,2-trifluoro-1,2-chloroethane-->1,2-DICHLORO-2-IODO-1,1,2-TRIFLUOROETHANE-->1,2-DICHLOROTETRAFLUOROETHANE-->1,1-DICHLORO-1,2-DIFLUOROETHANE-->TRIFLUOROETHYLENE-->1,2-Dibromo-1-chloro-1,2,2-trifluoroethane | | Preparation Products | FLUOROLUBE GREASE, GR-362-->2,3-Difluorotoluene-->2-(FLUOROSULFONYL)DIFLUOROACETYL FLUORIDE-->Pentafluoroethane-->1,2-DICHLOROTRIFLUOROETHANE-->Tetrafluoroethylene |
|



