|
| | Pyridinium chlorochromate Basic information |
| | Pyridinium chlorochromate Chemical Properties |
| Melting point | 205-208 °C(lit.) | | storage temp. | Inert atmosphere,Room Temperature | | solubility | Soluble in acetone, benzene, dichloromethane, acetonitrile and tetrahydrofuran. | | form | Crystalline Powder | | color | Orange | | Sensitive | Moisture Sensitive | | Merck | 14,7974 | | BRN | 7054643 | | Exposure limits | OSHA: Ceiling 0.1 mg/m3
NIOSH: IDLH 15 mg/m3; TWA 0.0002 mg/m3 | | Stability: | Stable. May react with easily oxidized materials. Incompatible with reducing agents, combustible material, metals, strong reducing agents. | | InChIKey | LEHBURLTIWGHEM-UHFFFAOYSA-N | | CAS DataBase Reference | 26299-14-9(CAS DataBase Reference) | | EPA Substance Registry System | Pyridinium chlorochromate (26299-14-9) |
| | Pyridinium chlorochromate Usage And Synthesis |
| Chemical Properties | Pyridinium chlorochromate is orange crystalline powder
| | Uses | Pyridinium chlorochromate is usually used to oxidize an allylic methylene group.
Oxidation of primary alcohols to aldehydes
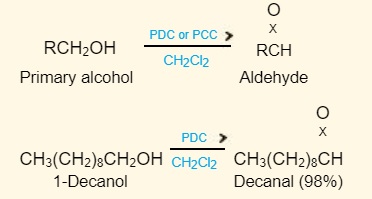
Pyridinium dichromate (PDC) or pyridinium chlorochromate (PCC) in anhydrous media such as dichloromethane oxidizes primary alcohols to aldehydes while avoiding overoxidation to carboxylic acids.
| | Uses | Oxidizing agent in organic synthesis.
PCC is pyridinium chlorochromate; PDC is pyridinium dichromate. Both are used in dichloromethane.
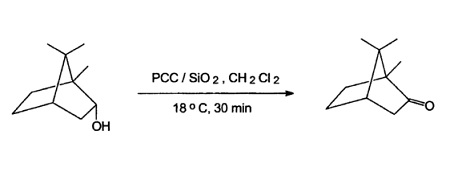
| | Uses | Pyridinium chlorochromate is used as an oxidizing agent to convert primary and secondary alcohols to aldehydes and ketones respectively. It is involved in the preparation of cyclohexanone, (-)-pulegone and lactones. It plays an important role in the selective mono-oxidation of xylenes to tolualdehydes and arylhydroxyamines to nitroso compounds. Furthermore, it is utilized as an oxidant for amino acids, L-cystine, aniline, cycloalkanols, vicinal and non-vicinal diols as well as in the Babler oxidation reaction. | | Reactions | Pyridinium chlorochromate (PCC) is a widespread used oxidation reagent but lacks of selectivity with unsaturated compounds. Furthermore, the workup procedure is quite tedious. Absorption of PCC onto silica gel improves the oxidation but then a reagent excess is necessary to reach appropriate rates. These drawbacks can be overcome by sonication leading to short reaction times and only an small excess of 1.2.-1.5 eq. of PCC is required. Using this reagent has been used to oxidise borneol to camphor in 85% yield.
| | Purification Methods | Dry it in a vacuum for 1hour. It is not hygroscopic and can be stored for extended periods at room temperature without change. If the purity is very suspect, it can be readily prepared. [Corey & Scuggs Tetrahedron Lett 2647 1975, Piancatelli et al. Synthesis 245 1982.] (Possible CARCINOGEN.) § Available commercially on a polymer support. |
| | Pyridinium chlorochromate Preparation Products And Raw materials |
|




