|
| | 4-Nitro-3-trifluoromethyl aniline Basic information |
| Product Name: | 4-Nitro-3-trifluoromethyl aniline | | Synonyms: | 4-Nitro-α,α,α;4-Nitro-3-(trifluoromethyl)aniline (FLU-1);5-Amino-2-nitrobenzotrifluoride, 4-Nitro-α,α,α-trifluoro-m-toluidine;4-nitro-3-(trifluoromethyl)benzenamine;FLU-1;4-Nitro-3-(trifluoromethyl)aniline ,98%;FlutaMide Related CoMpound A;The 4- nitro-3- threetrifluoroMethylaniline | | CAS: | 393-11-3 | | MF: | C7H5F3N2O2 | | MW: | 206.12 | | EINECS: | 206-884-6 | | Product Categories: | Anilines, Aromatic Amines and Nitro Compounds;Amines;Phenyls & Phenyl-Het;Phenyls & Phenyl-Het;Trifluoromethylbenzene serise;Amines, Aromatics, Metabolites & Impurities, Pharmaceuticals, Intermediates & Fine Chemicals;Aromatics;Intermediates & Fine Chemicals;Metabolites & Impurities;Pharmaceuticals;393-11-3 | | Mol File: | 393-11-3.mol |  |
| | 4-Nitro-3-trifluoromethyl aniline Chemical Properties |
| Melting point | 125-129 °C (lit.) | | Boiling point | 326.4±42.0 °C(Predicted) | | density | 1.4711 (estimate) | | storage temp. | 2-8°C | | solubility | DMSO (Slightly), Methanol (Slightly) | | pka | -0.22±0.10(Predicted) | | form | neat | | color | Yellow to Orange-Yellow | | BRN | 2650702 | | InChIKey | UTKUVRNVYFTEHF-UHFFFAOYSA-N | | CAS DataBase Reference | 393-11-3(CAS DataBase Reference) | | NIST Chemistry Reference | 5-Amino-2-nitrobenzotrifluoride(393-11-3) |
| | 4-Nitro-3-trifluoromethyl aniline Usage And Synthesis |
| Description | 4-Nitro-3-trifluoromethyl aniline is an organic intermediate, which can
be used to prepare 4-bromo-2-nitrotrifluorotoluene, an intermediate of
medicine and optical waveguide materials, and flutamide, a non steroidal
anti androgen drug. | | Chemical Properties | Orange to Brown to Dark red powder to crystal. | | Uses | 4-Nitro-3-(trifluoromethyl)aniline is a metabolite of Flutamide (F598850) (FLU-1 or M2). | | Uses | A metabolite of Flutamide (FLU-1 or M2). | | Synthesis | The
thiazolium salt 3 (3.30 mg, 0.0122 mmol) was added to a solution of
1-azido-4-nitrobenzene (0.122 mmol) and t-BuOH (18.0 mg, 0.244 mmol) in
degassed THF (0.50 mL) under argon at r.t. The resulting suspension was
stirred for 5 min, and then NaOt-Bu (23.4 mg, 0.244 mmol) was added in
one portion, and the mixture was stirred (Table 2). Water (2 mL) was
added, and the products were extracted with EtOAc (2 ?á 2 mL), the
combined organic phase was dried over anhyd MgSO4, filtered,
and concentrated in vacuo. Purification was achieved by passing the
resulting residue through a short pad of silica (eluting with 50% light
PE-EtOAc) unless otherwise stated.
4-Nitro-3-trifluoromethyl aniline, Time: 12 h, Pale yellow
solid (21.0 mg, 84%). (70:30 petrol-EtOAc) 0.5; mp 90-93 ??C (lit.,
92-93 ??C); IR (FTIR, CHCl3) |ímax cm-1: 3535 (NH2), 3434 (NH2), 1635, 1592 (NO2), 1520 (NO2), 1119; 1H NMR (400 MHz, DMSO-6) 8.59 (d, = 2.7 Hz, 1H), 8.55 (dd, = 9.3, 2.7 Hz, 1H), 7.55 (br s, 2H), 7.32 (d, = 9.3 Hz, 1H); 13C NMR (100 MHz, DMSO-6) 151.8 (C), 135.0 (C), 128.7 (CH), 123.7 (J = 5.9 Hz, F3N2NaO2 [M+Na]+, 229.0195; found, 229.0195.
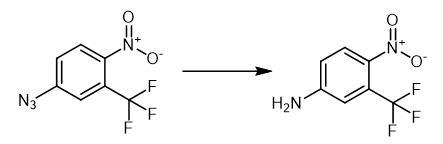
Fig. The synthetic of 4-Nitro-3-trifluoromethyl aniline |
| | 4-Nitro-3-trifluoromethyl aniline Preparation Products And Raw materials |
|



