|
| Product Name: | Ferric nitrate nonahydrate | | Synonyms: | IRON(+3)NITRATE ENNEAHYDRATE;IRON (III) NITRATE;IRON(III) NITRATE-9-HYDRATE;IRON(III) NITRATE ENNEAHYDRATE;IRON (III) NITRATE, HYDROUS;IRON(III) NITRATE NONAHYDRATE;IRON(III) NITRATE, NONOHYDRATE;FERRIC NITRATE 9H2O | | CAS: | 7782-61-8 | | MF: | FeH18N3O18 | | MW: | 404 | | EINECS: | 616-509-1 | | Product Categories: | metal nitrate salts | | Mol File: | 7782-61-8.mol |  |
| | Ferric nitrate nonahydrate Chemical Properties |
| Melting point | 47 °C(lit.) | | Boiling point | 125°C | | density | 1,68 g/cm3 | | Fp | 125°C | | storage temp. | Store at +5°C to +30°C. | | form | Solid | | color | White to gray or light purple | | Specific Gravity | 1.684 | | PH | 1.3 (100g/l, H2O, 20℃) | | Water Solubility | soluble | | Sensitive | Moisture Sensitive | | Merck | 14,4027 | | Exposure limits | ACGIH: TWA 1 mg/m3
NIOSH: TWA 1 mg/m3 | | Stability: | Stable. Strong oxidizer - contact with combustible material may lead to fire. Incompatible with combustible material, strong reducing agents, most common metals, organic material. May be light sensitive. | | CAS DataBase Reference | 7782-61-8(CAS DataBase Reference) | | EPA Substance Registry System | Ferric nitrate nonahydrate (7782-61-8) |
| | Ferric nitrate nonahydrate Usage And Synthesis |
| Description | Iron(III) Nitrate Nonahydrate is a highly water soluble crystalline Iron source for uses compatible with nitrates and lower (acidic) pH. All metallic nitrates are inorganic salts of a given metal cation and the nitrate anion. The nitrate anion is a univalent (-1 charge) polyatomic ion composed of a single nitrogen atom ionically bound to three oxygen atoms (Formula: NO3) for a total formula weight of 62.05. | | Uses | Ferric nitrate nonahydrate can be used as catalyst, mordant, metal surface treatment agent, oxidant, analytical reagent, radioactive substance adsorbent.
| | analysis method | Weigh 1~1.5g sample (accurate to 0.0001g) into a 250ml dry iodine measuring flask, and record the weight m. Add 20ml of water, 3g of potassium iodide, and 10ml of sulfuric acid solution (10%), and shake it up to dissolve completely, place in a dark place for 10-15 minutes, add about 80ml of water, titrate with sodium thiosulfate standard titration solution, and add when near the end 3ml starch indicator solution, continue to titrate until the blue color of the solution fades as the end point.
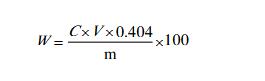
V--The value of the volume of sodium thiosulfate standard titration solution, in milliliter (mL);
c--The exact value of the concentration of sodium thiosulfate standard titration solution, the unit is moles per liter (mol/L);
0.404- -The mass of iron nitrate [(Fe (NO3) 3●9H2O)] equivalent to 1.00mL with sodium thiosulfate standard titration solution [C (Na2S2O3)=0.1000mol/L] in grams;
m---Sample mass, g.
| | Preparation | In the iron filing method, nitric acid with a relative density of 1.38 is added to an acid-resistant reactor filled with water, heated to 40-50°C, and fine iron filings are slowly added to react for 3 hours. The solution is heated to drive out the nitrogen oxide gas. Filter, evaporate and concentrate the filtrate to form a crystal film, send it to a cooling crystallizer to cool to below 0°C to precipitate crystals. After suction filtration, the crystals are washed with 20% nitric acid. The finished Ferric nitrate nonahydrate is produced. The reaction formula is as follows. Fe+4HNO3→Fe(NO3)3+NO+2H2OFe+6HNO3→Fe(NO3)3+3NO2+3H2O
The mother liquor can be recycled. The nitrogen oxide gas released during the reaction is commonly absorbed by caustic soda solution in industry. Because of its fast absorption rate, the obtained neutralization liquid is used to produce sodium nitrate.
| | Physical Properties | The nonahydrate form occurs as grayish-violet crystal; density 1.68 g/cm3; hygroscopic; decomposes at 47°C; very soluble in water, alcohol and acetone.
| | Uses | Ferric nitrate nonahydrate is used as a mordant for dyeing black and buff. Other applications are in tanning; weighting silks; and in preparation of analytical standards.
| | Preparation | Iron(III) nitrate is prepared by the action of nitric acid on iron filings or iron oxide followed by crystallization:
2Fe + 6HNO3 → 2Fe(NO3)3 + 3H2
Fe2O3 + 6HNO3 → 2Fe(NO3)3 + 3H2O
| | Chemical Properties | Pale purple solid | | Uses | Ferric nitrate nonahydrate is used in the determination of phosphate.
| | Uses | It is used as catalyst for the synthesis of sodium amide from sodium and ammonia, and for etching of silver and silver alloys. Ferric nitrate is used in the synthesis of organically templated iron phosphates, and acts as an efficient catalyst in enamination of beta dicarbonyl compounds to beta enaminones. Ferric nitrate impregnated clays are useful as oxidizing agents. Clayfen, produced from ferric nitrate and montmorillonite, is used for the oxidation of thiols to disulfide, and alcohols to aldehydes. Ferric nitrate is useful for regio- and stereo-selective nitration of various aromatic, aliphatic, and heteroaromatic olefins. This reaction provides nitroolefins with excellent E-selectivity. With Keggin-type heteropoly acids, it selectively oxidizes various hydroxy groups. | | General Description | This product has been enhanced for catalytic efficiency. | | Purification Methods | It crystallises from aqueous solutions of moderately strong HNO3 as the pale violet nonahydrate m 40o and is soluble in EtOH and Me2CO. With more concentrated aqueous solutions (containing some HNO3), the hexahydrate crystallises out m 60.5o . The anhydrous salt is slightly deliquescent and decomposes at 47o. [Lambert & Thomson J Chem Soc 97 2426 1920, Gmelin’s, Iron (8th edn) 59 Part B pp 161-172 1932.] |
| | Ferric nitrate nonahydrate Preparation Products And Raw materials |
|