|
| | Pyridinium dichromate Basic information |
| | Pyridinium dichromate Chemical Properties |
| Melting point | 152-153 °C(lit.) | | density | 1.71[at 20℃] | | vapor pressure | 0Pa at 25℃ | | storage temp. | Inert atmosphere,Room Temperature | | solubility | soluble in DMSO | | form | Crystalline Powder | | color | Orange to brown | | Water Solubility | soluble | | Sensitive | Hygroscopic | | Exposure limits | OSHA: Ceiling 0.1 mg/m3
NIOSH: IDLH 15 mg/m3; TWA 0.0002 mg/m3 | | InChIKey | BNSOYWDFFBDEFB-UHFFFAOYSA-L | | LogP | -3.7 at 20℃ | | CAS DataBase Reference | 20039-37-6(CAS DataBase Reference) | | EPA Substance Registry System | Chromic acid (H2Cr2O7), compd. with pyridine (1:2) (20039-37-6) |
| | Pyridinium dichromate Usage And Synthesis |
| Chemical Properties | Orange solid | | Uses | Pyridinium dichromate (PDC) is an orange colored solid used as an oxidizing agent.
Oxidizing agent for conversion of primary alcohols to aldehydes and ketones, acetals to esters, and didehydroketones to enones in the presence of tert-butyl hydroperoxide.
Pyridinium Dichromate may be used as an alternative to PCC in nucleoside and carbohydrate oxidation, particularly for fragile molecules. PDC can also be used in conjunction with tertbutylhydroperoxide for a variety of oxidative transformations. | | Uses | Pyridinium dichromate acts as a strong oxidizing agent used in the conversion of primary alcohols and secondary alcohols to aldehydes and ketones respectively. It plays an important role in the oxidation of unsaturated tertiary alcohols, silyl ethers, the carbon-boron bond, and oximes. Further, it is used in the conversion of acetals to esters and didehydroketones to enones in the presence of tert-butyl hydroperoxide. | | Definition | ChEBI: Pyridinium dichromate is a pyridinium salt that is the dipyridinium salt of dichromic acid. It is a strong oxidizing agent which can convert primary and secondary alcohols to aldehydes and ketones respectively. It has a role as an oxidising agent. It contains a dichromate(2-). | | Preparation | Pyridinium dichromate can be obtained by gradual addition of a solution of chromic anhydride (Cr2O3) in water to pyridine in ice cold conditions.
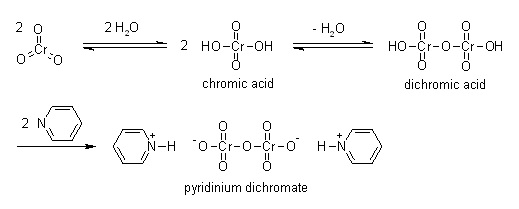
| | Flammability and Explosibility | Flammable | | Purification Methods | Dissolve it in the minimum volume of H2O and add 5 volumes of cold Me2CO and cool to -20o. After 3hours the orange crystals are collected, washed with a little cold Me2CO and dried in a vacuum. It is soluble in dimethylformamide (0.9g/mL at 25o), and in H2O, and has a characteristic IR with �max at 930, 875, 765, 730 and 730 cm-1. [Corey & Schmidt Tetrahedron Lett 399 1979, Coats & Corrigan Chem Ind (London) 1594 1969.] (Possible CARCINOGEN.) § Available commercially on a polymer support. |
| | Pyridinium dichromate Preparation Products And Raw materials |
|