|
| Product Name: | Dapoxetine | | Synonyms: | (S)-(+)-Dapoxetine;Dapoxetine-d7 HCl;Dapoxetine hydrochloride
(S-(+)-N,N-Dimethyl-a-[2-(naphthalenyloxy)ethyl]benzenemethanamine hydrochloride;Detoxetine;(S)-(+)-N,N-dimethyl-a-(2-(naphthalenyloxy)ethyl)benzenemethanamine;DAPOXETIN;DAPOXETINE;Dapoxetine hydrochloride | | CAS: | 119356-77-3 | | MF: | C21H23NO | | MW: | 305.41 | | EINECS: | 1308068-626-2 | | Product Categories: | Erectile Dysfunction;API;Dapoxetine hydrochloride;A | | Mol File: | 119356-77-3.mol |  |
| | Dapoxetine Chemical Properties |
| Melting point | 48 - 49°C | | Boiling point | 454.4±38.0 °C(Predicted) | | density | 1.081±0.06 g/cm3(Predicted) | | storage temp. | -20°C Freezer | | solubility | Chloroform (Slightly), DMSO (Slightly, Heated), Methanol (Slightly) | | pka | 8.38±0.50(Predicted) | | form | Solid | | color | White to Pale Beige | | CAS DataBase Reference | 119356-77-3(CAS DataBase Reference) |
| | Dapoxetine Usage And Synthesis |
| Brief Introduction | Premature ejaculation (PE) is the most common male sexual dysfunction. Dapoxetine hydrochloride,marketed as Priligy and Westoxetin, is a class of PE treatment drugs known as selective serotonin reuptake inhibitors (SSRI) , was the first drug originally approved for the on-demand treatment of men 18–64 years old with PE.
Dapoxetine works by inhibiting the serotonin transporter, increasing serotonin's action at the post synaptic cleft, and as a result promoting ejaculatory delay. Dapoxetine was initially developed as an antidepressant. However, unlike other SSRIs, dapoxetine is absorbed and eliminated rapidly in the body. Its fast acting property makes it suitable for the treatment of PE but not as an antidepressant.
Used for the treatment of premature ejaculation and erectile dysfunction. Over 98% of men taking this drug will naturally gain a improved erection quality with prolonged time and more frequencies. | | Physical and chemical properties | White crystal powder with sweet taste and no odor, hygroscopic and soluble in water. It has a stable chemical property with the melting point at 175~177℃. | | PE treatment | Premature ejaculation (PE) is a common problem worldwide and has significant impact not only on the sufferer but on the partner in terms of self-esteem, interpersonal distress and sexual satisfaction. Tricyclic antidepressants and clomipramine are the most widely used drugs for the treatment of premature ejaculation .
Randomized, double blind, placebo-controlled trials have confirmed the efficacy of dapoxetine for the treatment of PE. Different dosage has different impacts on different type of PE. Dapoxetine 60 mg significantly improves the mean intravaginal ejaculation latency time (IELT) compared to that of dapoxetine 30 mg in men with lifelong PE, but there is no difference in men with acquired PE. Dapoxetine, giving 1–3 hours before sexual episode, prolongs IELT, increases the sense of control and sexual satisfaction in men of 18 to 64 years of age with PE. Since PE is associated with personal distress, interrelationship difficulty, dapoxetine provides help for men with PE to overcome this condition. Because lack of specific approval treatment for PE in the US and some other countries, other SSRIs such as fluoxetine, paroxetine, sertraline, fluvoxamine, and citalopram have been used as off label drugs to treat PE. Waldinger's meta analysis shows that the use of these conventional antidepressants increasing IELT from two to ninefold above base line in comparison of three to eightfold when dapoxetine is used. However, these SSRIs must be taken daily in order to achieve meaningful efficacy, and the long half-life increases the risk of the drug accumulation and as a consequence increased of adverse effects such as decreasing sexual libido and causing erectile dysfunction. Dapoxetine, on the other hand, is a fast-acting SSRI. It is rapidly absorbed and eliminated from the body within a few hours. This favorable pharmacokinetics minimizes the risk of the drug's accumulation in the body, and therefore reducing side effects. | | Mechanism of Actions | The mechanism through which dapoxetine affects premature ejaculation is still unclear. However, it is presumed that dapoxetine works by inhibiting serotonin transporter and subsequently increasing serotonin's action at pre and postsynaptic receptors. Human ejaculation is regulated by various areas in the central nervous system (CNS). The ejaculatory pathway originates from spinal reflex at the thoracolumbar and lumbosacral level of spinal cord activated by stimuli from male genital. These signals are relayed to the brain stem, which then is influenced by a number of nuclei in the brain such as medial preoptic and paraventricular nulcei. Clement's study performed on anaesthetized male rats showed that acute administration of dapoxetine inhibits ejaculatory expulsion reflex at supraspinal level by modulating activity of lateral paragigantocellular nucleus (LPGi) neurons. These effects cause an increase in pudendal motoneuron reflex discharge (PMRD) latency. However, it is unclear whether dapoxetine acts directly on LPGi or on the descending pathway in which LPGi located. | | Development in Marketing internationally | Originally developed by Eli Lilly pharmaceutical company, dapoxetine was sold to Johnson & Johnson in 2003 and submitted as a New Drug Application to the Food and Drug Administration (FDA) for the treatment of PE in 2004.Dapoxetine is sold in some European and Asian countries, and in Mexico. In the US, dapoxetine has been in phase III development since 2003. However, it was rejected by FDA last year. In 2012, Menarini acquired the rights to sell Dapoxetine in Europe, most of Asia, Africa, Latin America and the Middle East. | | Synthesis | There are many ways to synthesize dapoxetine, which can be divided into two categories. The first category is achiral synthesis where pure S-configuration dapoxetine is obtained from the resolution of racemic dapoxetine or its intermediate. The other is the methods of asymmetric synthesis, where S-configuration dapoxetin is directly obtained through metal catalysis or enzyme catalysis without resolution .
- Achiral synthesis methods
(1) Synthesis of dapoxetine with benzaldehyde as the starting material: benzylaldehyde, ammonium acetate and malonic acid are reacted by Mannich reaction and then N-methylated, esterified and reduced to ether to obtain the racemic product. The starting material, benzaldehyde and malonic acid, are cheap and readily available, but the yield of compound 2 is low, and the expensive LiAlH4 and material are required in the synthesis process.
(2) Synthesis of dapoxetine from 1-naphthol as raw material
The reaction between 1-naphthol and 3-phenylpropyl bromide is followed by bromination on the benzyl radical, the substitution of dimethylamine and the resolution of the obtained racemic products. The starting material of this route, 3-phenylpropyl bromide is rather expensive, and the bromination yield is low. 3-phenylpropyl bromide can also be replaced by 3-phenylpropyl chloride obtained by the reaction of phenylpropanol with dichlorofuran.
(3) Synthesis of dapoxetine from (s)-3-chlorophenyl propanol as raw material
the etherification between (S)-3-chlorophenyl propanol and 1-naphthol is followed by the one-pot reaction of methanesulfonyl chloride and dimethylamine to get S configuration of dapoxetine. This route has relatively less step and no need for resolution but the raw material (S)-3-chloropropenal alcohol is expensive and often unavailable and the reaction conditions are demanding.
(4) Synthesis of dapoxetine from 3-chlorophenylacetone as raw material
Through the reduction, condensation and N-methylation of 3-chlorophenylacetone as the starting material, the racemic dapoxetine is obtained and then its S-configuration is obtained. But the expensive 3-chlorophenyl acetone causes high production costs.
- Asymmetric synthesis methods
(1) Synthesis of dapoxetine with N-Boc-(R)-phenylglycine as raw material
In this routine, N-Boc-(R)-phenylglycine 13, the carboxyl group is reduced to hydroxyl and the hydroxy is sulfonylated to produce the intermediate 14. And the addition of sodium cyanide is carried out to extend the carbon chain , And the known compound 10 is obtained after the reduction of the carboxylic acid. Finally, the target product, dapoxetine, was obtained by a two-step reaction. The raw material N-Boc-(R)-phenylglycine is not readily available and its price is high. The use of expensive borane and highly toxic NaCN makes the long synthesis route less welcomed.
(2) Synthesis of dapoxetine with (R)-1-phenyl-1,3-propanediol as raw material
The primary hydroxyl group in (R)-1-phenyl-1,3-propanediol 15 is selectively protected with p-toluenesulfonyl chloride, etherified, methanesulfonylated and amino-substituted to produce dapocetine. The chiral raw material 15 source is difficult to get with a high price. | | Usage and Dosage | Oral administration of one pill 20-30 mins before sexual intercourse; it can be taken irregularly; it’s better to take it with an empty stomach; avoid fatty food during administration; no tea before administration. Its effect is significant when the user under sexual stimulus. | | Adverse effects | The most common effects when taking dapoxetine are nausea, dizziness, dry mouth, headache, diarrhea, and insomnia, which will soon disappear. Discontinuation due to adverse effects is dose related. According to McMahon in recent study in Asia, the rate of discontinuation is 0.3%, 1.7%, and 5.3% of 1067 studied subjects with placebo, dapoxetine 30 mg, and dapoxetine 60 mg respectively. Unlike other SSRIs used to treat depression, which have been associated with high incidences of sexual dysfunction, dapoxetine is associated with low rates of sexual dysfunction. Taken as needed, dapoxetine has very mild adverse effects on loss of libido (<1%) and ED (<4%). | | Contraindications | A contraindication is a situation in which a drug should not be used, because it may be harmful to the patient. Juveniles, children and pregnant women are not allowed to take dapoxetine. Dapoxetine should not be used in men with moderate to severe hepatic impairment and in those receiving CYP3A4 inhibitors such as ketoconazole, ritonavir, and telithromycine. Dapoxetine can also not be used in patients with heart failure, permanent pacemaker, or other significant ischemic heart disease. Caution is advised in men receiving thioridazine, monoamine oxidase inhibitors, SSRIs, serotonin-norepinephrine reuptake inhibitors, or tricyclic antidepressant. If a patient stops taking one of these drugs, he should wait for 14 days before taking dapoxetine. If a patient stops taking dapoxetine, he should wait for 7 days before receiving these drugs. | | Interactions |
- With phosphodiesterase inhibitors (PDE5 inhibitors)
Many men that have PE also suffer from erectile dysfunction (ED). Treatment for these patients should consider the drug-drug interaction between dapoxetine and PDE5 inhibitors such as tadalafil (Cialis) or sildenafil (Viagra). In Dresser study (2006), plasma concentration of 24 subjects was obtained. Half of the sample pool were treated with dapoxetine 60 mg + tadalafil 20 mg; the other half were treated with dapoxetine 60 mg + sildenafil 100 mg. These plasma samples were then analyzed using liquid chromatography-tandem mass spectrometry. The results showed that dapoxetine does not alter the pharmacokinetic of tadalafil or sildenafil.[2]
Ethanol doesn't affect the pharmacokinetics of dapoxetine when taking concurrently with dapoxetine. | | References | http://www.nature.com/articles/srep07269
https://en.wikipedia.org/wiki/Dapoxetine | | Uses | Antidepressant. | | Uses | Dapoxetine-d7 Hydrochloride is a Labelled Dapoxetine (D185700). A selective serotonin reuptake inhibitor (SSRI). | | Definition | ChEBI: Dapoxetine is a member of naphthalenes. | | Clinical Use | Dapoxetine is a selective serotonin re-uptake inhibitor (SSRI)
which has been approved in Finland and Sweden for the treatment
of premature ejaculation.Dapoxetine was discovered and developed
by Lilly and was licensed to Alza, a wholly-owned subsidiary
of Johnson & Johnson. | | Synthesis | Several synthetic routes for the preparation of dapoxetine have been disclosed, all on gram scale. Based on
the routes and yields, the most likely process route is described
in the scheme 4. Commercially available (R)-(+)-3-chloro-1-phenyl-
1-propanol (19) was reacted with 1-naphthol in the presence of
50% aqueous sodium hydroxide in DMF to give ether 20 in 90%
yield. Activation of the secondary alcohol was accomplished
through treatment of 20 with methane sulfonyl chloride and triethylamine
with catalytic DMAP. Upon complete conversion to
the corresponding mesylate, dimethylamine was added to the
reaction mixture. The addition of hydrochloric acid in ethyl acetate
to the resultant crude product gave dapoxetine hydrochloride (IV)
in 67% yield. Purification of this material by re-crystallization from
isopropanol provided IV in 86% yield and in 99.6% ee. 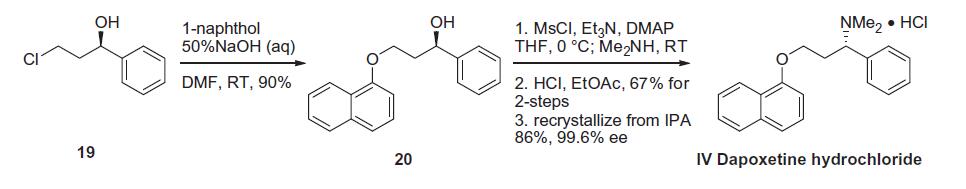
|
| | Dapoxetine Preparation Products And Raw materials |
|