| | 3-Chloroperoxybenzoic acid Chemical Properties |
| Melting point | 69-71 °C(lit.) | | Boiling point | 244.67°C (rough estimate) | | density | 0.56 | | vapor pressure | 0.373Pa at 25℃ | | refractive index | 1.4580 (estimate) | | storage temp. | 2-8°C | | solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) | | pka | 7.57 (in water @ 25 °C) | | form | Moist Powder | | color | White | | Odor | slight pungent odor | | PH | 4.5@25 °C (saturated aq. sol) | | Water Solubility | insoluble | | Decomposition | >88 °C | | BRN | 608317 | | Stability: | Strong oxidizing agent - contact with combustible material may cause fire. May be shock or heat sensitive. Incompatible with organic materials, strong reducing agents. | | InChIKey | NHQDETIJWKXCTC-UHFFFAOYSA-N | | LogP | 1.03 at 25℃ | | CAS DataBase Reference | 937-14-4(CAS DataBase Reference) | | NIST Chemistry Reference | 3-Chloroperbenzoic acid(937-14-4) | | EPA Substance Registry System | Benzenecarboperoxoic acid, 3-chloro- (937-14-4) |
| | 3-Chloroperoxybenzoic acid Usage And Synthesis |
| Chemical Properties | 3-chloroperoxybenzoic acid is white powdery crystals. Melting point is 92-94°C (decomposed). It is almost insoluble in water, but soluble in ethanol, ethers, chloroform, and dichloroethane. It is thermally stable and has an annual decomposition rate of less than 1% at room temperature. The decomposition rate is accelerated in the liquid state. 3-Chloroperbenzoic acid is sensitive to heat and shock, and pure solid. 3-Chloroperbenzoic acid is flammable and potentially explosive. It contains a weak –O–O– bond and a nucleophilic OH group, that makes it versatile oxidative and easily breakable. | | Uses | 3-Chloroperoxybenzoic acid is commonly used in double bond epoxidation, nitridation, cyclization, Baeyer-Villiger oxidation, and N-oxidation. It can also be used as an oxidant for fine chemicals such as synthetic medicine and pesticides. It is also sometimes used as a bleaching agent [1-6].
• Used in cyclization reaction, Baeyer-Villiger reaction, N-oxidation reaction and S-oxidation reaction.
• Used as an oxidant for fine chemical products such as synthetic medicine and pesticides.
• Used as oxidant and bleach.
• As a good electrophilic reagent, it can react with many functional groups and can oxidize olefins, enol silyl ethers, furans, sulfides, selenides and amino compounds.
| | Chemical Properties | White moist powder | | Uses | 3-Chloroperoxybenzoic acid is a strong oxidizing agent used in the oxidation reactions such as aldehydes and ketones to esters (Bayer-Villiger-Oxidation), olefines to epoxides, sulfides to sulfoxides and sulfones, and amines to nitroalkanes, N-oxides. | | Uses | Effective oxidant for epoxidizing di-, tri-, and tetra-substituted olefins. | | Definition | ChEBI: 3-chloroperbenzoic acid is a peroxy acid and a member of monochlorobenzenes. | | Reactions | 3-Chloroperoxybenzoic acid (MCPBA) is one of the most popular oxidation reagent in organic synthesis, because of its outstanding performance in terms of:
reactivity, combined with reducing the number of reaction steps in classical synthetic routes,
regio- and stereoselectivity,
protection of functional groups mostly not required,
high purity and yields.
Its literature covers a huge area of different syntheses and below reaction equations just can be a brief overview of its interesting applications:
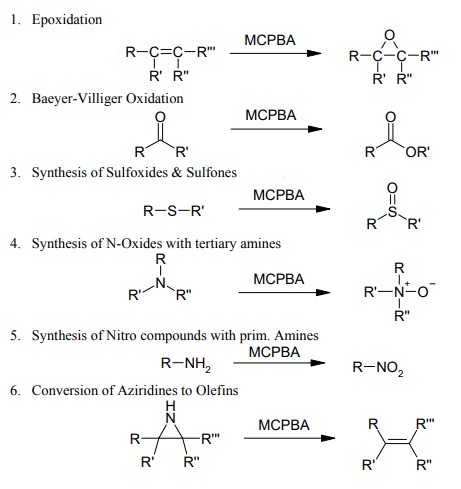 | | Synthesis Reference(s) | Synthetic Communications, 19, p. 1271, 1989 DOI: 10.1080/00397918908054534 | | General Description | 3-Chloroperoxybenzoic acid is sensitive to heat. Storage of 3-Chloroperoxybenzoic acid must be done so with stringent temperature control measures. It's explosion hazard is also mitigated by mixing the peroxide in a solvent slurry. | | Reactivity Profile | May explode from heat, shock, friction or contamination. May ignite combustibles (wood, paper, oil, clothing, etc.). May be ignited by heat, sparks or flames. | | Purification Methods | Recrystallise MCPBA from CH2Cl2 [Traylor & Mikztal J Am Chem Soc 109 2770 1987]. Peracid of 99+% purity can be obtained by washing commercial 85% material with phosphate buffer pH 7.5 and drying the residue under reduced pressure. Alternatively the peracid can be freed from m-chlorobenzoic acid by dissolving 50g/L of *benzene and washing with an aqueous solution buffered at pH 7.4 (NaH2PO4/NaOH) (5 x 100mL). The organic layer is dried over MgSO4 and carefully evaporated under vacuum. Necessary care should be taken in case of EXPLOSION. The solid is recrystallised twice from CH2Cl2/Et2O and stored at 0o in a plastic container as glass catalyses the decomposition of the peracid. The acid is assayed iodometrically. [Schwartz & Blumbrgs J Org Chem 29 1976 1964, Bortolini et al. J Org Chem 52 5093 1987, McDonald et al. Org Synth Coll Vol VI 276 1988, Beilstein 9 IV 972.] |
| | 3-Chloroperoxybenzoic acid Preparation Products And Raw materials |
| Raw materials | 1,4-Dioxane-->Magnesium sulfate heptahydrate-->Polyethylene-->3-Chlorobenzoyl chloride | | Preparation Products | 3,5-DIMETHYLPYRIDIN-4-AMINE-->2-(2,3-DIHYDRO-1-BENZENESULFONYL-PYRROLO[2,3-B]PYRIDIN-3-YL)ACETONITRILE-->4,5-DICHLORO-2-METHYLPYRIDINE-->METHYL(2R,3S)-2,2-DIMETHYL-3-(2-OXOPROPYL)-CYCLOPROPANEACETATE-->2,5-Dichloropyridine-->1-PROPANESULPHONYLACETONITRILE-->2-Hydroxymethyl-5-bromopyridine-->5-Bromopyridine-2-carbaldehyde-->3-AMINOMETHYL-PYRIDINE-2-CARBOXYLIC ACID-->4-(CHLOROSULFONYL)-7-FLUORO-2,1,3-BENZOXADIAZOLE-->4-Chloro-2-(methylsulfonyl)pyrimidine-->S-METHYL-S-(2-METHYLPYRAZINYL) SULFOXIMINE-->4-[(TERT-BUTOXYCARBONYLAMINO)METHYL]-2-CYANOPYRIDINE-->4-(BOC-AMINOMETHYL)PYRIDINE-2-CARBOXYLIC ACID-->4-FLUORO-2,1,3-BENZOXADIAZOLE-->LORACARBEF (200 MG)-->1,4-OXATHIANE SULFOXIMINE-->3-(TERT-BUTOXYCARBONYLAMINO-METHYL)-PYRIDINE-2-CARBOXYLIC ACID-->Omeprazole-->Tacalcitol-->8-HYDROXYQUINOLINE-2-CARBONITRILE-->5-BROMO-4-CHLORO-2-METHANESULFONYL-PYRIMIDINE-->1,2-EPOXYHEXANE-->1,7-DIDEAZAADENINE-->1H-PYRROLO[2,3-B]PYRIDINE, 4-NITRO--->5-BROMO-2-METHYLPYRIDINE N-OXIDE-->4-Bromo-7-azaindole-->NITROCYCLOHEXANE-->1,2-EPOXY-9-DECENE-->6-BROMO-1H-PYRROLO[2,3-B]PYRIDINE-->6-CHLORO-1H-PYRROLO[2,3-B]PYRIDINE-->3-ISOCHROMANONE-->4-(METHYLSULFINYL)PHENOL-->1H-Pyrrolo[2,3-b]pyridine, 4-nitro-, 7-oxide-->4-IODO-7-AZAINDOLE-->4-Chloro-7-azaindole-->Cyclopentene oxide-->Pantoprazole-->3-NITROSOBENZAMIDE-->7-OXIDE-7-AZAINDOLE |
|



