|
| | OLANEXIDINE Basic information |
| Product Name: | OLANEXIDINE | | Synonyms: | OLANEXIDINE;Imidodicarbonimidic diamide, N-((3,4-dichlorophenyl)methyl)-N'-octyl;Olanexidine [inn];N-[(3,4-Dichlorophenyl)methyl]-N'-octylimidodicarbonimidic diamide;N1-(3,4-Dichlorobenzyl)-N5-octylbiguanide;Olanexidine Gluconate;N''-(3,4-dichlorobenzyl)-N'''-octylimidodicarbonimidic diamide;1-[N'-[(3,4-dichlorophenyl)methyl]carbamimidoyl]-2-octylguanidine | | CAS: | 146510-36-3 | | MF: | C17H27Cl2N5 | | MW: | 372.34 | | EINECS: | | | Product Categories: | | | Mol File: | 146510-36-3.mol |  |
| | OLANEXIDINE Chemical Properties |
| Boiling point | 454.7±55.0 °C(Predicted) | | density | 1.22±0.1 g/cm3(Predicted) | | pka | 11.92±0.10(Predicted) |
| | OLANEXIDINE Usage And Synthesis |
| Description | In July 2015,
olanexidine gluconate, a biguanide compound with remarkable
antibacterial activity, was approved by the Pharmaceuticals and
Medical Devices Agency (PMDA) of Japan for skin antisepsis
at surgical sites. The drug was developed and marketed by
Otsuka Pharmaceutical in Japan and is available as topical
solution (1.5%). Olanexidine gluconate exhibited efficacy
against a wide range of bacterial strains, especially Grampositive
bacteria. In vitro experiments exploring its mechanism
of action indicated that olanexidine interacts with bacterial
surface molecules (such as lipopolysaccharides and lipoteichoic
acid), disrupting the cell membranes of liposomes. These
models suggest that the drug permeates the membranes of both
Escherichia coli and Staphylococcus aureus and denatures proteins
at relatively high concentrations (>160 g/mL). | | Definition | ChEBI: Olanexidine is a dichlorobenzene. | | Synthesis | The synthesis of olanexidine gluconate is relatively
straightforward, involving the linkage of an n-octyl side chain
and a dichlorobenzylamine through a bis-guanidyl lynchpin.
The synthesis began with the reaction of commercial noctylamine
(17) with sodium dicyanamide in the presence of
concentrated sulfuric acid in refluxing n-butyl acetate to give
rise to 1-cyano-3-octylguanidine (18) in 86% yield .
Conditions employed to subsequently secure biguanidine 20 as
the HCl salt hemihydrate in 77% yield were nearly identical to
those used for the conversion of 17 to 18. Finally, treatment of
20 with sodium hydroxide in the presence of gluconic acid (21)
gave rise to olanexidin gluconate (II) in almost quantitative
yield. 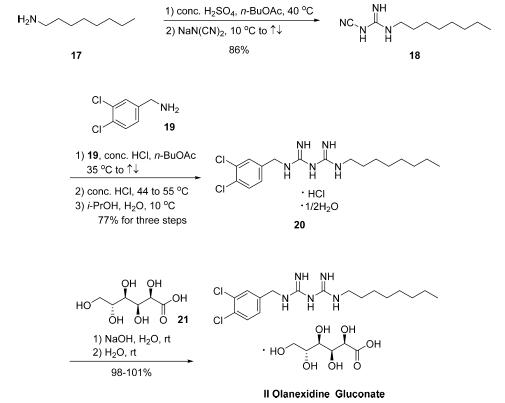
|
| | OLANEXIDINE Preparation Products And Raw materials |
|