|
| | Rupatadine Fumarate Basic information |
| Product Name: | Rupatadine Fumarate | | Synonyms: | Rupafin FuMarate;Rupax FuMarate;5H-Benzo[5,6]cyclohepta[1,2-b]pyridine, 8-chloro-6,11-dihydro-11-[1-[(5-methyl-3-pyridinyl)methyl]-4-piperidinylidene]-, (2E)-2-butenedioate (1:1);RUPATADINE FUMARATE;8-chloro-6,11-dihydro-11-[1-[(5-methyl-3-pyridyl)methyl]-4-piperidylidene]-5h-benzo[5,6]cyclohepta[1,2-b]pyridine fumarate;Alergoliber FuMarate;Pafinur FuMarate;Rinialer FuMarate | | CAS: | 182349-12-8 | | MF: | C26H26ClN3.C4H4O4 | | MW: | 532.03 | | EINECS: | 1592732-453-0 | | Product Categories: | Inhibitors;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 182349-12-8.mol |  |
| | Rupatadine Fumarate Chemical Properties |
| Melting point | 196-198°C | | storage temp. | Sealed in dry,2-8°C | | solubility | Methanol (Slightly, Heated) | | form | Solid | | color | White to Pale Brown |
| | Rupatadine Fumarate Usage And Synthesis |
| Description | Rupatadine fumarate, a novel antiallergic drug with a dual mechanism of action, was
introduced in Spain as an oral treatment for perennial and seasonal rhinitis.
Rupatadine acts as non-sedating histamine H1 receptor antagonist and plateletactivating
factor (PAF) antagonist. Its Kiapp
i values against [3H]WEB-2086 binding to
rabbit platelet membrane PAF receptors and [3H]pyralimine binding to guinea pig
cerebellum membrane H1 histamine receptors are 0.55 and 0.10 μM, respectively. It
has a rapid onset of action, with patients experiencing relief of symptoms within 2 h,
and its long duration of action (>24 h) permits once-daily dosing. Rupatidine is
prepared in a 6-step convergent synthesis, with the key steps involving the Grignard
reaction of a N-alkyl-4-chloropiperdine with a benzocycloheptapyridinone intermediate,
followed by dehydration. Rupatadine is rapidly absorbed after oral administration.
The time to reach maximum plasma concentration is 0.75–1 h and the mean
half-life in healthy volunteers is ~6 h. It is extensively metabolized, mainly by
CYP3A4, and the major elimination route for the drug is biliary excretion. In
comparative clinical trials, rupatadine 10 mg once daily was as effective as certizine
10 mg in short-term studies (2–4 weeks duration), but provided a better profile of
CNS side effects. In comparison with ebastine 10 mg and loratadine 10 mg,
rupatadine showed a superior relief of rhinitis symptoms at the same dose.
Rupatadine was well tolerated in clinical trials and, at the recommended daily dose
of 10 mg, was free of the sedative effects associated with first-generation
antihistamines. In addition, there were no significant differences in the overall
incidence of adverse events in rupatidine-treated patients and those treated with
placebo or standard reference products. | | Chemical Properties | Rupatadine fumarate is white or off-white color crystalline powder, odorless, and mildly bitter flavor, slightly draws moistly, in methanol, dissolves, almost insoluble in water, slightly molten in 0.1mol/L hydrochloric acid solution. | | Originator | Uriach (Spain) | | Uses | Rupatadine is a dual antagonist of histamine H1 and platelet-activating factor receptors. Rupatadine is used as an antihistaminic. | | Uses | 8-Chloro-6,11-dihydro-11-[1-[(5-methyl-3-pyridyl)methyl]-4-piperidylidene]-5H-benzo[5,6]cyclohepta[1,2-b]pyridine fumarate is a dual antagonist of histamine H1 and platelet-activating factor receptors. Rupatadine is used as an antihistaminic.
| | Brand name | Rupafin | | Synthesis | One of the convergent
syntheses for rupatadine (XX) involved two key
intermediates, tricyclic ketone 162 and chloropiperidine
derivative 167. 3-Methylpicoline acid (157) was reacted with
p-chloroaniline in the presence of acid chloride and TEA to
provide amide 158 in 91% yield. Amide 158 was then treated with n-BuLi at -20??C for 1h, followed by addition of
3-chlorobenzyl chloride (159) to furnish amide 160 in 91%
yield after an aqueous workup. The cyclization of amide 160
was accomplished by treatment with 160 PCl5 first followed
by AlCl3 mediated Friedel-Crafts cyclization. The cyclic
intermediate 161 was directly subjected to hydrolysis
without isolation and tricyclic ketone 162 was obtained in
71% yield via a one-pot process. N-acylation of 5-
hydroxypiperidine (164) with 5-methylnictonic acid (163)
was accomplished by using HOBT, DCC to furnish amide
165. The carbonyl group in 165 was reduced by
chlorination/reduction sequence using POCl3 and NaBH4.
Alcohol 166 was then converted to the chloride 167 by
refluxing with SOCl2 in CHCl3. Coupling tricyclic ketone
162 and chloride 167 via a Grinard protocal followed by
dehydration furnished the rupatadine 168. Treatment of
rupatadine with fumaric acid in EtOH gave rupatadine
fumarate (XX) in 70% yield. 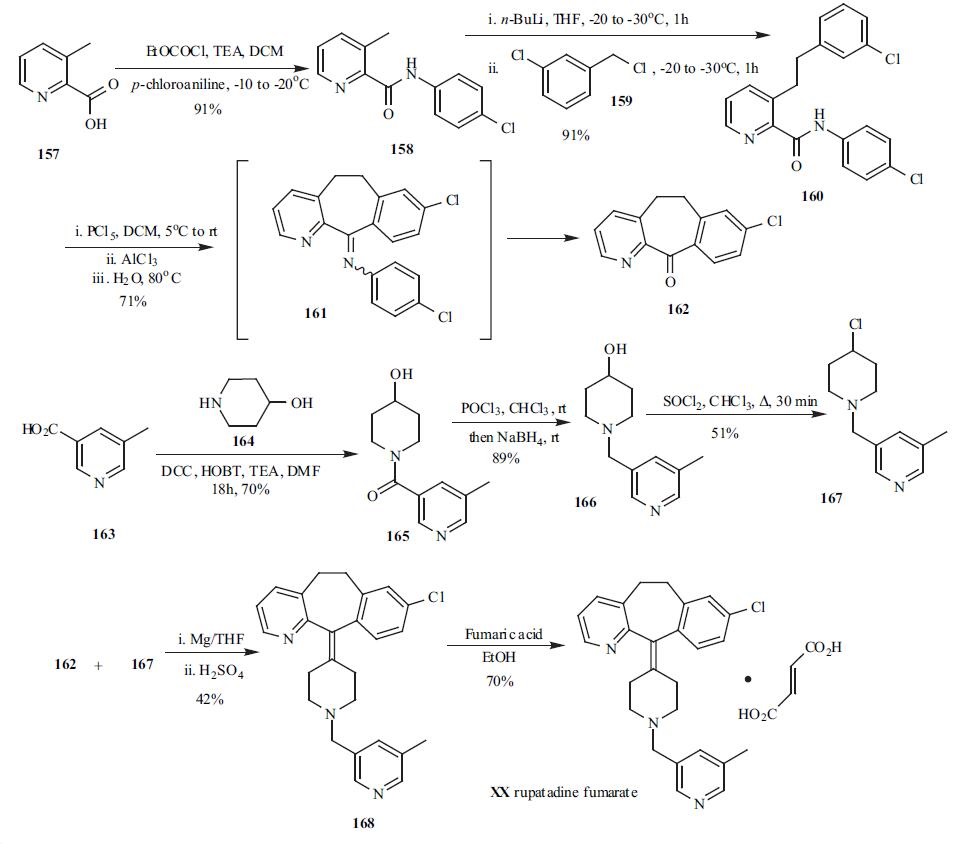
|
| | Rupatadine Fumarate Preparation Products And Raw materials |
|