|
| | Silodosin Chemical Properties |
| Melting point | >104°C (dec.) | | alpha | D25 -14.0° (c = 1.01 in methanol) | | Boiling point | 601.4±55.0 °C(Predicted) | | density | 1.249±0.06 g/cm3(Predicted) | | storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C | | solubility | Aqueous Base (Slightly, Heated Sonicated), DMSO (Slightly, Sonicated), Methanol | | pka | 14.85±0.10(Predicted) | | form | Solid | | color | White to Off-White | | CAS DataBase Reference | 160970-54-7(CAS DataBase Reference) |
| | Silodosin Usage And Synthesis |
| Treatment of prostatic hyperplasia | Silodosin is a kind of α-adrenergic receptor antagonists developed by Japanese Kissei pharmaceutical company. It has a very good therapeutic efficacy on treating the dysuria related to benign prostatic hyperplasia. The selective effect on urethra of Silodosin is 12 times and 7.4 times as high as that of prazosin and tamsulosin, respectively, which can significantly inhibit norepinephrine-induced contraction of the prostate; it has dose-dependent inhibition bladder activity excitation in the benign prostatic hypertrophy models of rats, and can also improve the pressure threshold of bladder contraction. These data have suggested that, in addition to helping improve bladder function, silodosin is also effective in alleviating the related symptoms associated with benign prostatic hypertrophy.
Compared with similar drugs such as prazosin and tamsulosin, silodosin has a high selectivity onn theα1A-receptors located in the prostate and bladder neck, while having a low affinity to theα1B-and α1D-receptor. It can block theα1A-receptors in these sites, and relax the smooth muscle, and thus leading to the improvement of urinary flow rate and the alleviation of BPH symptoms.
The selective binding to the α1A-receptor by silodosin has a higher selectivity compared to the binding to the cardiovascular associated receptors α1B, thus maximizing the activity of the target organ as well as minimizing the potential effect on blood pressure.
The above information is edited by the chemicalbook of Dai Xiongfeng.
| | Benign prostatic hyperplasia | Benign prostatic hyperplasia (referred as BPH) is one of the most common diseases in older male with the non-malignant prostate being the character. It can cause the obstructive urination symptoms and other irritation symptoms which have brought significant negative impact on the life of older people. In recent years, with the increasing level of industrialization of China, the annual intake of animal protein intake gradually increased and life expectancy increases, the incidence of benign prostatic hyperplasia also has increased year by year. Over 50 percent in elderly people at age 60 or above has got this disease. This ratio increases to over 90% for elderly people over 85 years-old. Its triggering factor has not been totally elucidated. It is generally believed that this is related to the secretion of sex hormones and cholesterol as well as other kinds of metabolic disorders. Some people think that, for elder people, their weakened pituitary-gonadotropin-pathway or endogenous change cause degeneration of testicles decreased sexual function, reduced testosterone value, as well as the change of glandular epithelium and enlarged prostate caused by the increased connected tissue of prostate.
The enlargement of prostate will compress the urethra, resulting in poor urine flow of bladder and even blocked bladder outlet. There is α1A-adrenergic receptor located in the human prostate. The activation of the receptor will deteriorate the symptoms of dysuria and urethral obstruction. Therefore, by blocking the binding α1A-adrenergic receptor, we can relax the obstructed prostate smooth muscle relaxation, and thereby alleviating the symptoms. In recent years, people has achieved excellent efficacy by clinically applying 5α-reductase inhibitors and α1-adrenergic receptors (α1-AR) blocker (silodosin). This is considered as a major breakthrough in drug treatment of benign prostatic hyperplasia.
| | Uses | It can be used for treatment of benign prostatic hyperplasia.
| | Description | Silodosin, an a1A adrenoceptor (a1A-AR) antagonist selective for prostatic
receptors, was launched as an oral treatment for dysuria associated with
benign prostatic hypertrophy (BPH). The regulation of smooth muscle tone in the
bladder neck and prostate is thought to be primarily mediated by a1A-AR.
Blockade of these receptors can cause smooth muscle relaxation in these areas,
resulting in improved symptoms and urinary flow rates. Conversely, a1B-AR are
largely located on vascular smooth muscle, and antagonism of these receptors
can cause tissue relaxation and potentially decrease cardiac compensation mechanisms
involved in regulating blood pressure. | | Chemical Properties | White Solid | | Originator | Kissei (Japan) | | Uses | Silodosin is an α1a-adrenoceptor antagonist. It is used in treatment of benign prostatic hypertrophy. | | Uses | An α1a-adrenoceptor antagonist. It is used in treatment of benign prostatic hypertophy. | | Uses | Silodosin(Rapaflo) is an α1-adrenoceptor antagonist with high uroselectivity. It causes practically no orthostatic hypotension (in contrast to other α1 blockers). Since Silodosin is a highly selective inhibitor of the α1A adrenergic receptor, it causes pr | | Definition | ChEBI: Silodosin is an indolecarboxamide. | | Brand name | Urief | | Synthesis | The synthesis of silodosin
has been disclosed in several patents. The latest
synthetic route disclosed in the 2006 patent is highlighted
in the scheme. The synthesis started with Grignard generation from readily available bromoindoline 65 by treating
it with Mg in the presence of a catalytic dibromoethane
in THF. After initiation of the reaction with some heat and
refluxing at a steady rate, CBZ protected oxazolidinone 66
[39b] was added over 1 h, refluxed for 4 h and then stirred at
room temperature for 2 days. The reaction was quenched
with 6 M aqueous HCl and stirred for 12 h after which time
the reaction was worked up to provide product 67 in 53%
yield. Ketone 67 was then treated with triethylsilane in TFA
at 0oC and stirred at room temperature for 10 h to provide
amine 68 in 61% yield. Bromination of the indoline 68 with
bromine in warm acetic acid furnished bromide 69 in 53%
yield which was reacted with copper cyanide in DMF at
130oC to give the cyano indoline 70 in 82% yield. Selective
deprotection of the benzyloxycarbonyl over the benzyl group
was accomplished by reacting indoline 70 with 1 atm hydrogen
in the presence of 5% Pd/C in ethanol at room temperature.
The resulting free amine 71 was then reacted with mesylate
72 in t-butanol with sodium carbonate as base at
80-90oC for 46 h to provide 73 in 67% yield. Removal of the
benzyl ether was accomplished by reacting 73 with 1 atm
hydrogen in the presence of 10%Pd/C to give alcohol 74,
which upon hydrolysis provided the desired silodosin (X).
No yield for the final reaction was given. 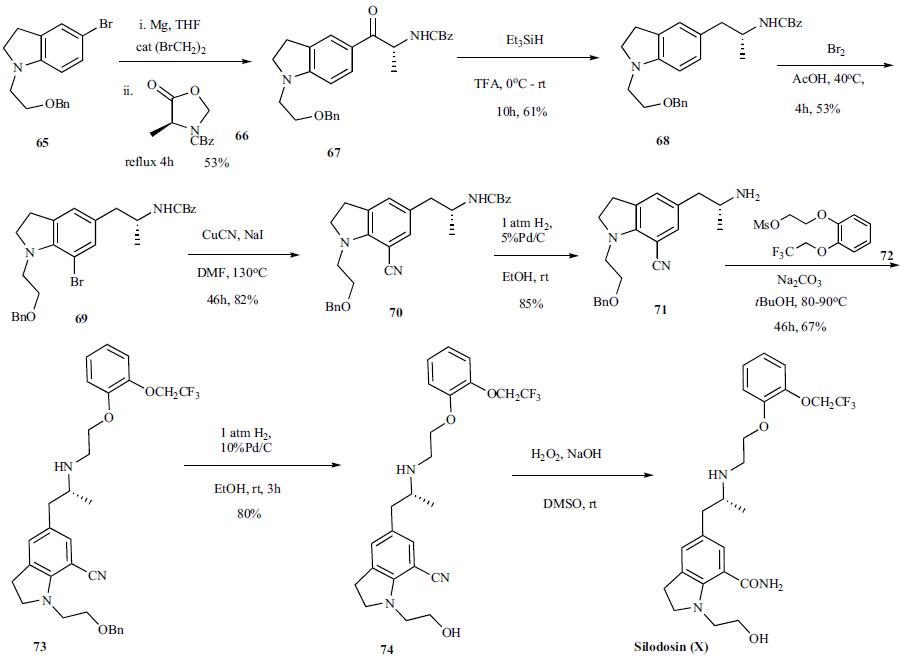
| | target | Adrenergic Receptor | | storage | Store at -20°C |
| | Silodosin Preparation Products And Raw materials |
|



