|
| | Chloro(1,5-cyclooctadiene)iridium(I) dimer Basic information | | Reactions |
| Product Name: | Chloro(1,5-cyclooctadiene)iridium(I) dimer | | Synonyms: | BIS(1,5-CYCLOOCTADIENE)DIIRIDIUM(I) DICHLORIDE;DI-MU-CHLOROBIS[(ETA-CYCLOOCTA-1,5-DIENE)IRIDIUM (I)];DI-MU-CHLORO-BIS[(1,2,5,6-ETA)-1,5-CYCLOOCTADIENE]DIIRIDIUM;CHLORO(1,5-CYCLOOCTADIENE)IRIDATE (I) DIMER;CHLORO(1,5-CYCLOOCTADIENE)IRIDIUM(I) DIMER;IRIDIUM(I) CHLORIDE 1,5-CYCLOOCTADIENE COMPLEX DIMER;IRIDIUM I CYCLOOCTADIENE CHLORIDE;IRIDIUM CHLORO-1,5-CYCLOOCTADIENE | | CAS: | 12112-67-3 | | MF: | C16H24Cl2Ir2 | | MW: | 671.7 | | EINECS: | 235-170-7 | | Product Categories: | Ir;organometallic complexes;Ir (Iridium) Compounds;Catalysts for Organic Synthesis;Classes of Metal Compounds;Homogeneous Catalysts;Metal Complexes;Synthetic Organic Chemistry;Transition Metal Compounds | | Mol File: | 12112-67-3.mol |  |
| | Chloro(1,5-cyclooctadiene)iridium(I) dimer Chemical Properties |
| Melting point | 205 °C (dec.)(lit.) | | storage temp. | Keep in dark place,Inert atmosphere,2-8°C | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | Powder | | color | red to orange | | Water Solubility | insoluble | | Hydrolytic Sensitivity | 7: reacts slowly with moisture/water | | InChIKey | XHOSESNLNGITPM-XRGHXPOKSA-L |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-37/39 | | WGK Germany | 3 | | F | 10 | | TSCA | No | | HS Code | 28439000 |
| | Chloro(1,5-cyclooctadiene)iridium(I) dimer Usage And Synthesis |
| Reactions | 1. Precursor to catalysts for the asymmetric hydrogenation of tri- and tetrasubstituted olefins.
2. Precursor to catalyst for enantioselective reduction of imines.
3. Precursor to catalyst for allylic alkylation.
4. Precursor to catalyst for allylic amination and etherification.
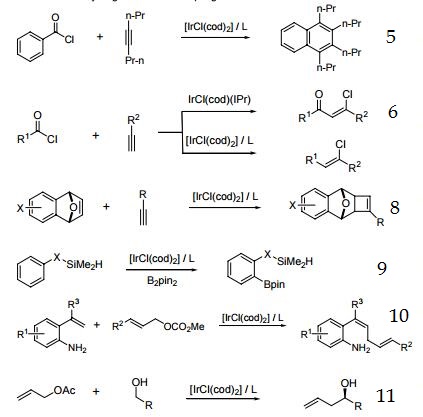
5. Precursor to catalyst for the reaction of aroyl chlorides with internal alkynes to produce substituted naphthalenes and anthracenes.
6. Ir-catalyzed addition of acid chlorides to terminal alkynes.
7. Intramolecular hydroamination of unactivated alkenes with secondary alkyl- and arylamines.
8. Enantioselective [2+2] cycloaddition.
9. Silyl-directed, Ir-catalyzed ortho-borylation of arenes.
10. Ir-catalyzed cross-coupling of styrene derivatives with allylic carbonates.
11. Transfer hydrogenative C-C coupling
| | Chemical Properties | red-orange solid | | Uses | Chloro(1,5-cyclooctadiene)iridium(I) dimer is widely used as a precursor to other iridium complexes, which finds application in homogeneous catalysis like carbonylation, hydrosilylation, hydrofomylation, asymmetric allylic substitutions, metathesis and chiral catalysis reactions. It is involved in the preparation of Crabtree's catalyst, which is used for hydrogenation and hydrogen-transfer reactions. |
| | Chloro(1,5-cyclooctadiene)iridium(I) dimer Preparation Products And Raw materials |
|



