| Chemical Properties | Allylpalladium(II) chloride dimer is a yellow powder with the formula [(η3-C3H5)PdCl]2. This compound is an important catalyst used in organic synthesis. It is one of the most widely used transition metal allyl complexes. |
| Uses | Allylpalladium(II) chloride dimer is used as an important catalyst in Heck reaction. It reacts with cyclopentadienyl anion to give cyclopentadienyl allyl palladium. It acts as a precatalyst for asymmetric and cross-coupling catalysis. Further, it is used in the preparation of 1,4-diallyl-1,2-dihydroisoquinolines. It is also employed as a catalyst for greener Buchwald-Hartwig coupling reaction and involved in the synthesis of cationic palladium catalysts. In addition, it takes part in preparation of -heterocyclic carbene-palladium-eta3-allyl chloride complex, which is an efficient catalyst for the Suzuki-Miyaura cross-coupling reactions in synthetic chemistry. |
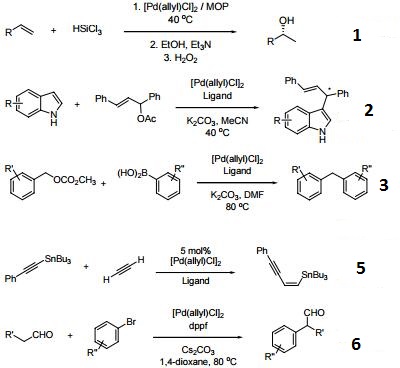
| Uses | [Pd(allyl)Cl]2 can be used as a catalyst:
For the silylation of organobromides.
To synthesize α-aryl carbonyl compounds by coupling reaction between aldehydes and aryl halides.
To prepare various arylthiophene derivatives by cross-coupling reaction with aryl halides via C-H functionalization.
Application Guide for Palladium Catalyzed Cross-Coupling Reactions
ChemDose: Convenient Dosing of Catalysts and Reagents |
| Preparation | Allylpalladium(II) chloride dimer is prepared by purging carbon monoxide through a methanolic aqueous solution of palladium(II) chloride, sodium chloride, and allyl chloride.
2Na2PdCl4 + 2 CH2=CHCH2Cl + 2 CO + 2 H2O → (C3H5)2Pd2Cl2 + 4 NaCl + 2 CO2 + 4 HCl |
| Reactions |
-
Precatalyst for the enantioselective hydrosilylation of olefins.
-
Precatalyst for asymmetric allylic alkylation and amination.
-
Used as a palladium source for cross-coupling reactions.
-
Can be used with Trost ligands.
-
Catalyst for the carbostannylation of alkynes.
-
Used as a precatalyst for "-arylation of aldehydes.
|
| General Description | Allylpalladium(II) chloride dimer is employed as catalyst in Heck reaction. It also participates as catalyst in the tandem nucleophilic allylation-alkoxyallylation reaction of the alkynylaldehydes with allyl chloride and allyltributylstannane. |
| Purification Methods | It crystallises from benzene and is soluble in MeOH, Et2O and CHCl3. [Hüttel et al. Chem Ber 94 766 1961, Dent et al. J Chem Soc 1585 1964, Armstrong J Org Chem 31 618 1966.] |