|
| | O-(2,4-dinitrophenyl)hydroxylamine Basic information |
| | O-(2,4-dinitrophenyl)hydroxylamine Chemical Properties |
| Melting point | 112.5°C | | Boiling point | 336.62°C (rough estimate) | | density | 1.6937 (rough estimate) | | refractive index | 1.6910 (estimate) | | storage temp. | 2-8°C(protect from light) | | solubility | DMSO (Sparingly), Methanol (Slightly) | | pka | -1.07±0.70(Predicted) | | form | solid | | color | yellow |
| Risk Statements | 22-36/37/38 | | Safety Statements | 26 | | TSCA | No | | HS Code | 29221990 |
| | O-(2,4-dinitrophenyl)hydroxylamine Usage And Synthesis |
| Chemical Properties | Light yellow solid | | Uses | Efficient agent for metal-free amination of arylboronic acids leading to primary anilines. Reagents used in Rhodium-catalyzed aziridines formation. | | Uses | O-(2,4-Dinitrophenyl)hydroxylamine is a rapid active-site-directed inhibitor of D-amino acid oxidase; modification results in specific incorporation of an amine group into an accessible nucleophilic r
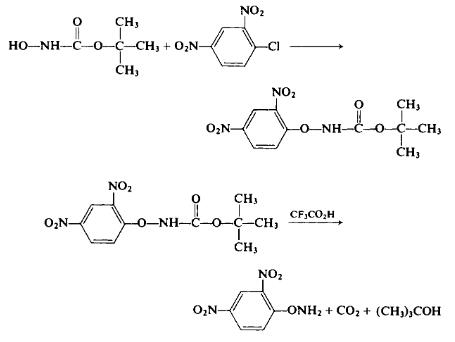
esidue with concomitant release of 2,4-dinitrophenol. | | Preparation | To a stirred solution of 13.3 gm (0.1 mole) of t-butyl JV-hydroxycar-bamate and 5.6 gm (0.1 mole) of potassium hydroxide in 200 ml of absolute ethanol is added 20.2 gm (0.1 mole) of 2,4-dinitrochlorobenzene. The resultant deep red solution is stirred at room temperature for 1 hr; then enough glacial acetic acid is added dropwise to produce a light yellow solution. The solution is poured into 1.5 liters of cold water. The yellow oil which separates is gradually converted to crystals. The solid ?-butyl JV-(2,4-dinitrophenoxy)carbamate is separated, dried, and recrystallized from an ethyl acetate-hexane mixture to afford 16.4 gm (53%), m.p. 74-75°C. To 15 ml of trifluoroacetic acid is added 4 gm (0.0133 mole) of the i-butyl J/V-(2,4-dinitrophenoxy)carbamate. After the evolution of carbon dioxide has subsided, the solution is poured into 100 ml of ice water. The resultant oily layer crystallizes on standing to afford 2.5 gm (95%), m.p. 112°C (from ethanol).
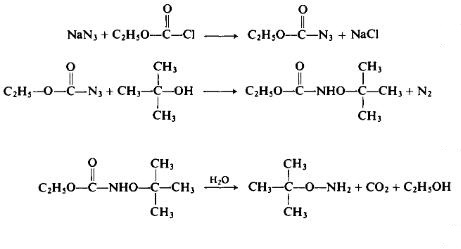
Recently it was discovered that the alkylation of ethyl JV-hydroxy-carbamate under alkaline conditions, particularly in a DMF medium at 60°C in the presence of sodium bicarbonate, leads to the ultimate formation of O-alkylated hydroxylamines. On the other hand, at 80-85°C, the direct alkylation without the presence of a base ultimately leads to N-alkylhydrox-ylamines (see Table I) [59]. The reaction of ethylazidoformate with an alcohol, while perhaps hazardous, may have some merit (Eqs. 31-33). The overall yield, based on ethyl chloroformate, is said to be on the order of 60%.
 |
| | O-(2,4-dinitrophenyl)hydroxylamine Preparation Products And Raw materials |
|